All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Venetoclax with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma
Venetoclax, an oral B-cell lymphoma 2 (BCL-2) inhibitor, has been studied in combination with other agents in the treatment of multiple myeloma (MM), particularly in the setting of relapsed or refractory (RR) disease. The latest results from the BELLINI trial (NCT02755597) investigating venetoclax combined with the proteasome inhibitor bortezomib and dexamethasone demonstrated significantly higher median progression-free survival compared with the control arm; click here for the visual abstract summarizing data presented at 63rd American Society of Hematology Annual Meeting and Exposition.
Carfilzomib, a new proteasome inhibitor, causes apoptosis of MM cells, and indirectly promotes BCL-2 dependency in MM cells.1 Given the previous success of venetoclax in combination with other agents that increase BCL-2 dependency, Luciano Costa and colleagues published the results of their phase II open-label dose-escalation trail (NCT02899052) of venetoclax plus carfilzomib and dexamethasone (VenKd) in patients with RRMM in Blood Advances.1 The study is summarized in this article.
Study design
- Patients received treatment with VenKd at one of four dose-finding levels or as part of the expansion cohort (Figure 1) until disease progression or intolerable adverse events (AEs).
- Of note, following the results of the BELLINI study in which higher rates of infection and infection-related death were observed in the venetoclax plus bortezomib/dexamethasone arm, antibiotic prophylaxis was implemented in this study.
Figure 1. Treatment regimens*†
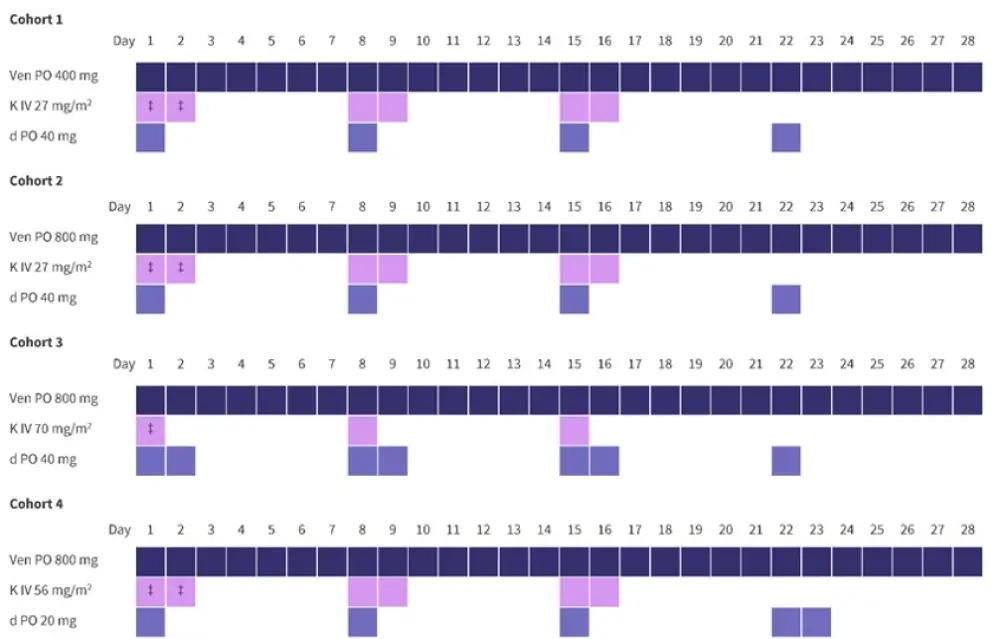
d, dexamethasone; IV, intravenous; K, carfilzomib; PO, oral; Ven, venetoclax.
*Adapted from Costa et al.1
†PO and IV fluids were given ≥72 hours prior to the first dose of venetoclax and carfilzomib. Prophylactic antibiotics and antivirals were also given.
‡Carfilzomib was administered at 20 mg/m2 during the first week and was increased to the target dose in Cycle 1 on Day 8.
Baseline characteristics
Patients ≥18 years of age with RRMM were eligible for the study, with inclusion criteria including 1–3 prior lines of therapy, an Eastern Cooperative Oncology Group performance status ≤2, and measurable disease. Patients who had received carfilzomib previously and those with disease refractory to any BCL-2 inhibitor, significant cardiovascular disease, or Grade ≥3 peripheral neuropathy were excluded. Baseline characteristics are shown in Table 1.
Table 1. Baseline characteristics*
|
*Adapted from Costa et al.1 |
|||
|
Characteristic, % (unless otherwise specified) |
All patients |
t(11;14) |
Non-t(11;14) |
|---|---|---|---|
|
Median age (range), years |
66 (37–79) |
63 (47–75) |
68 (37–79) |
|
Race |
|
|
|
|
White |
80 |
77 |
81 |
|
Black or African American |
20 |
23 |
19 |
|
ECOG PS |
|
|
|
|
0 |
31 |
31 |
31 |
|
1–2 |
59 |
54 |
61 |
|
Missing |
10 |
15 |
8 |
|
ISS stage |
|
|
|
|
I |
37 |
62 |
29 |
|
II |
31 |
8 |
39 |
|
III |
31 |
31 |
31 |
|
Unknown |
2 |
0 |
3 |
|
Cytogenetic status |
|
|
|
|
High-risk† |
27 |
31 |
25 |
|
Standard-risk‡ |
59 |
69 |
56 |
|
Unknown |
14 |
0 |
19 |
|
Cytogenetic abnormalities |
|
|
|
|
t(11;14) |
27 |
100 |
0 |
|
t(4;14) |
4 |
0 |
6 |
|
t(14;16) |
4 |
0 |
6 |
|
del(17p) |
20 |
31 |
17 |
|
1q21 gain (≥3 copies) |
43 |
39 |
44 |
|
Hyperdiploid |
22 |
15 |
25 |
|
Median time from diagnosis to treatment (range), months |
37.4 (2.4–195.6) |
37.7 (4.9–126.7) |
34.7 (2.4–195.6) |
|
Median prior lines of therapy (range) |
1 (1–3) |
1 (1–2) |
1 (1–3) |
|
Refractory to last prior therapy |
86 |
69 |
92 |
|
Prior stem cell transplantation |
51 |
23 |
61 |
|
Prior exposure to PI |
96 |
92 |
97 |
|
Refractory to PI |
57 |
69 |
53 |
|
Prior exposure to IMiD |
90 |
77 |
94 |
|
Refractory to IMiD |
71 |
69 |
72 |
|
Prior exposure to PI + IMiD |
86 |
69 |
92 |
|
Double refractory |
45 |
46 |
44 |
|
High BCL-2 expression (IHC)§ |
93 |
100 |
90 |
|
High BCL-2 expression (qPCR)‖ |
56 |
89 |
47 |
Results
Overall, 20 patients were recruited to the four dose-finding cohorts and a further 29 were included in the expansion cohort. Most patients received 70 mg/m2 carfilzomib (71%) and 800 mg venetoclax (92%). The maximum tolerated dose was not reached.
Safety
- No patients in the dose-finding cohorts experienced a dose-limiting toxicity.
- All patients experienced ≥1 treatment-emergent adverse event (TEAE), and 92% of patients experienced Grade 3 or 4 TEAEs. The most common any grade and Grade ≥3 TEAEs are shown in Table 3.
- Serious TEAEs (defined as AEs that were life threatening, required medical intervention to prevent serious outcome, or resulted in significant disability or death) occurred in 53%, which included pneumonia (14%), influenza (6%), acute kidney injury (4%), congestive cardiac failure (4%), and hypoxia (4%).
- Rates of infection were similar to those observed with venetoclax monotherapy.
- Five deaths occurred, including in two patients due to Grade 5 infections (non-treatment-emergent pulmonary aspergillosis and treatment-emergent influenza B), one patient due to respiratory arrest, one due to an unexplained cause possibly related to carfilzomib, and one due to non-treatment-emergent disease progression.
Table 3. A summary of the most common TEAEs in all patients*
|
*Adapted from Costa et al.1 |
||
|
TEAE, % |
Any grade |
Grade ≥3 |
|---|---|---|
|
Hematologic |
|
|
|
Lymphopenia |
35 |
31 |
|
Leukopenia |
22 |
12 |
|
Neutropenia |
22 |
12 |
|
Infections |
|
|
|
URTI |
39 |
0 |
|
Sinusitis |
20 |
0 |
|
Pneumonia |
18 |
12 |
|
Influenza† |
16 |
6 |
|
Nonhematologic |
|
|
|
Diarrhea |
65 |
10 |
|
Fatigue |
47 |
6 |
|
Nausea |
47 |
4 |
|
Hypertension |
27 |
16 |
Efficacy
Response rates and minimal residual disease negativity in all patients, as well as according to important patient subgroups, are shown in Figure 2 and Table 4.
Figure 2. ORR and best overall response in all patients and key subgroups*
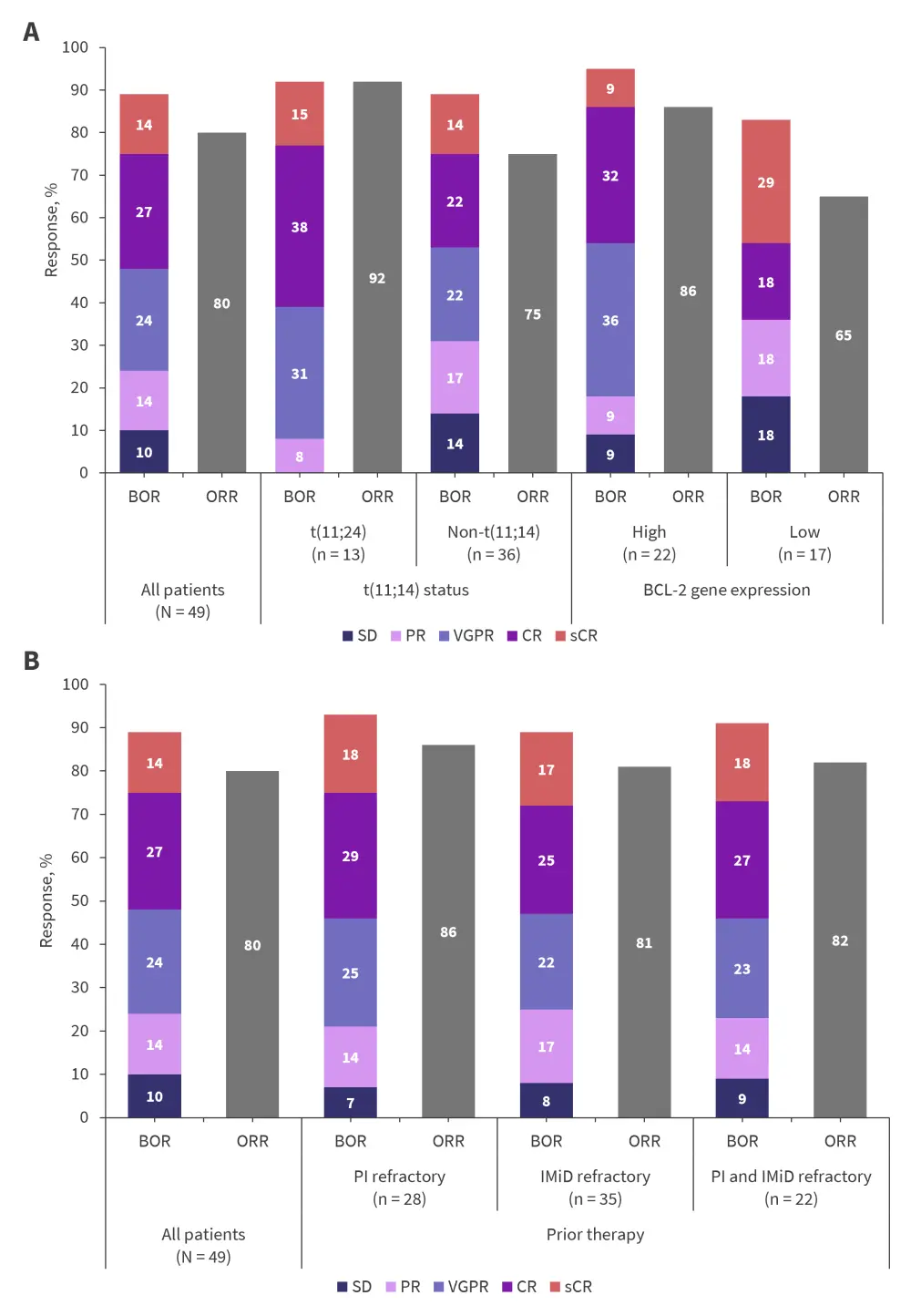
Best overall responses and overall response rates in all patients and depending on A t(11;14) status, BCL-2 gene expression, and B prior therapy.
BCL2, B-cell lymphoma 2; BOR, best overall response; CR, complete response; IMiD, immunomodulatory drug; ORR, overall response rate; PI, proteasome inhibitor; PR, partial response; sCR, stringent CR; SD, stable disease; VGPR, very good partial response.
*Data from Costa et al.1
Table 4. MRD negativity, DOR, and PFS in all patients and key subgroups*
|
BCL-2, B-cell lymphoma 2; CI, confidence interval; DOR, duration of response; IMiD, immunomodulatory drug; MRD, minimal residual disease; NE, not evaluable; PFS, progression-free survival; PI, proteasome inhibitor. |
||||||||
|
|
All patients |
t(11;14) |
Non-t(11;14) |
BCL-2 gene expression |
Prior therapy |
|||
|---|---|---|---|---|---|---|---|---|
|
High |
Low |
PI refractory |
IMiD refractory |
PI and IMiD refractory |
||||
|
MRD, %† |
|
|
|
|
|
|
|
|
|
<10−4 |
18 |
31 |
14 |
32 |
12 |
21 |
20 |
23 |
|
<10−5 |
12 |
15 |
11 |
18 |
12 |
11 |
11 |
9 |
|
<10−6 |
4 |
8 |
3 |
9 |
0 |
4 |
3 |
5 |
|
Median DOR, months (CI) |
19.7 (13.1–NE) |
NR |
16.4 (9.2–NE) |
23.8 (19.8–NE) |
16.4 (13.2–NE) |
19.7 (9.2–NE) |
16.4 |
NR |
|
Median PFS, months (CI) |
22.8 (12.4–NE) |
24.8 (8.1–NE) |
22.8 (9.5–NE) |
24.7 (9.0–NE) |
22.8 (3.7–NE) |
— |
— |
— |
Conclusion
The novel combination of VenKd in patients with RRMM demonstrated promising, durable responses, with generally superior outcomes observed in patients with t(11;14) and high BCL-2 gene expression.
The safety profile was similar to that observed in trials with carfilzomib monotherapy and carfilzomib combination therapy with dexamethasone, as well as triplet therapy with the further addition of daratumumab. Rates of cytopenia were slightly higher in this study compared with other combination studies of carfilzomib, likely due to the venetoclax AE profile. This aside, it appears the addition of venetoclax to carfilzomib and dexamethasone did not increase treatment toxicity.
This study further supports a biomarker-driven approach for venetoclax treatment in MM; however, given the limited sample size and lack of randomization, further investigation with prospective clinical studies of VenKd is warranted.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

