All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
UVEA-IXA: Efficacy and safety of ixazomib-based therapy outside a clinical trial setting
Do you know... The early access program UVEA-IXA investigated the response of ixazomib in patients diagnosed with relapsed/refractory multiple myeloma across Europe outside of a clinical trial setting. What was the overall response rate to ixazomib in patients who had received ≥1 prior line of therapy?
The oral proteasome inhibitor ixazomib was approved in combination with lenalidomide and dexamethasone in November 2015 by the U.S. United States Food and Drug Administration (FDA) for patients diagnosed with multiple myeloma (MM) who had received ≥1 prior line of therapy. This approval was based on results collected from the phase III TOURMALINE-MM1 trial (NCT01564537). Between the time of the US approval date and the subsequent European Medicines Agency (EMA) approval in November 2016, ixazomib was made available across Europe via an early access program, UVEA-IXA (Use Via Early Access to Ixazomib).
The program aimed to describe the effect of ixazomib in patients with relapsed/refractory (RR) MM across Europe, outside of a clinical trial setting. Ludwig et al.1 presented the final results of the program in Clinical Lymphoma, Myeloma and Leukemia. We are pleased to summarize the key findings below.
Study design1
- Multicenter, observational, noninterventional, longitudinal cohort study
- Split into two phases:
-
- A retrospective chart review
- A 1-year prospective observation period
- The primary objectives were assessing best response and progression-free survival
Results1
- A total of 309 evaluable patients were enrolled, across 43 sites and eight countries
- The median observation period was 25.5 months
- Baseline patient characteristics at study enrollment are shown in Table 1 and baseline characteristics at treatment initiation are shown in Table 2.
Table 1. Baseline patient characteristics at study enrollment*
|
*Adapted from Ludwig, et al.1 |
|
|
Characteristic, % (unless otherwise stated) |
N = 309 |
|---|---|
|
Median age, years |
68 |
|
Male |
54 |
|
Prior lines of therapy |
|
|
1 |
38 |
|
2 |
43 |
|
3 |
18 |
|
Race |
|
|
White |
98 |
|
Other |
2 |
Table 2. Baseline patient characteristics at treatment initiation*
|
ECOG PS, European Cooperative Oncology Group performance scale; ISS, International Staging System. |
|
|
Characteristic, % (unless otherwise stated) |
N = 123† |
|---|---|
|
ECOG PS score |
|
|
0 |
24 |
|
1 |
56 |
|
2 |
20 |
|
ISS stage |
|
|
I |
35 |
|
II |
35 |
|
III |
30 |
|
Cytogenetic risk |
|
|
High-risk |
30 |
|
No or standard risk |
70 |
Efficacy
- The overall response rate, median progression-free, and median overall survival for all patients and those with ≥1 and ≥2 prior lines of therapy are shown in Figure 1.
Figure 1. A Overall response rate, B median PFS, and C median overall survival*
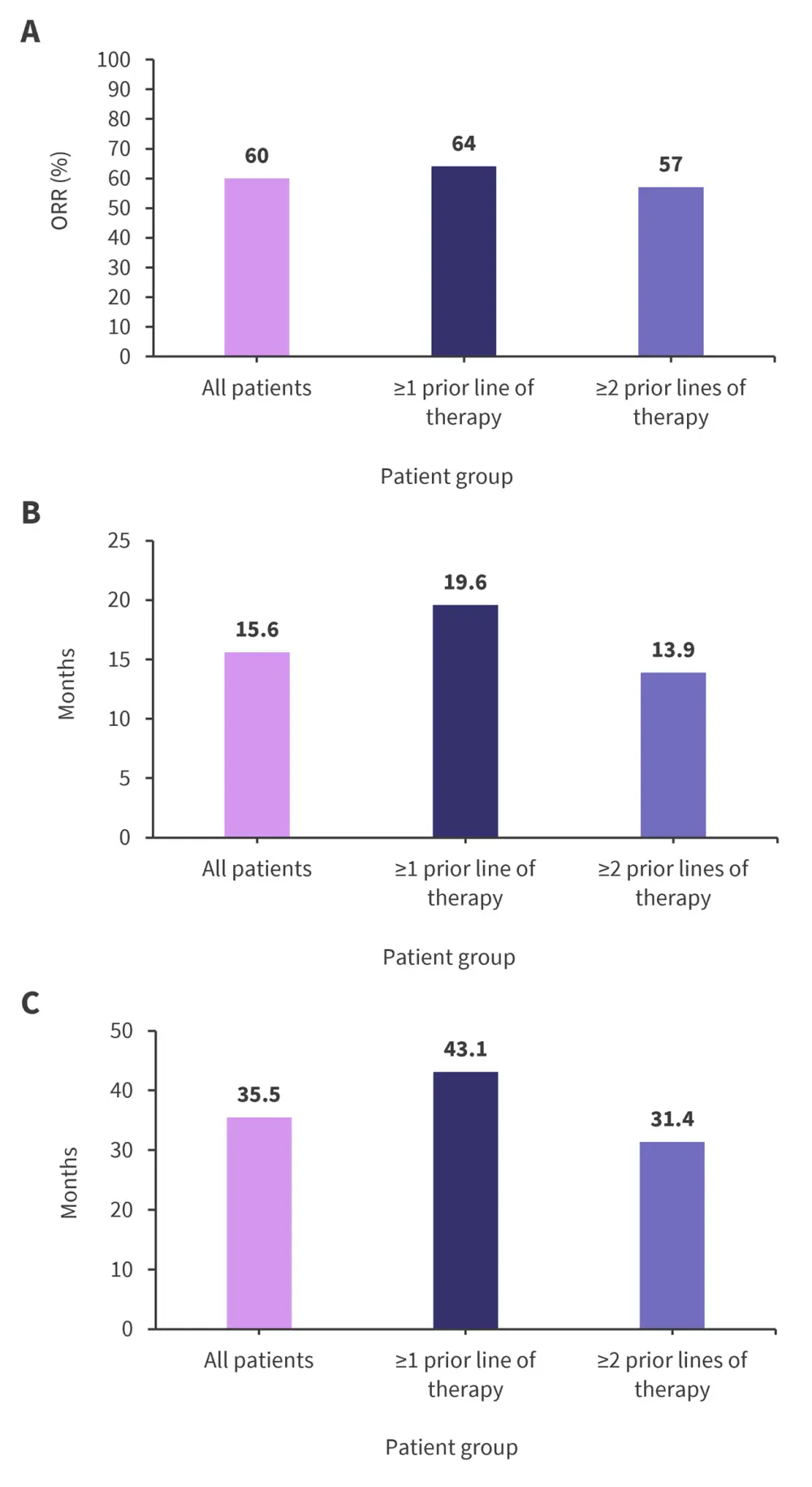
ORR, overall response rate; PFS, progression-free survival.
*Adapted from Ludwig et al1
- A total of 55% of patients received subsequent therapy after ixazomib
- The median time to the next therapy was 21.4 months
Safety
- Overall, 18% of patients required dose reductions of ixazomib, and 34% dose reductions in lenalidomide
- Ixazomib was discontinued in 80% of patients
- Adverse events (AEs) of any grade were experienced by 64% of patients
- Grade ≥3 AEs were experienced by 37% of patients
- Serious AEs were experienced by 33% of patients
- The most common AEs of any grade are shown in Figure 2
Figure 2. Most common AEs of any grade*
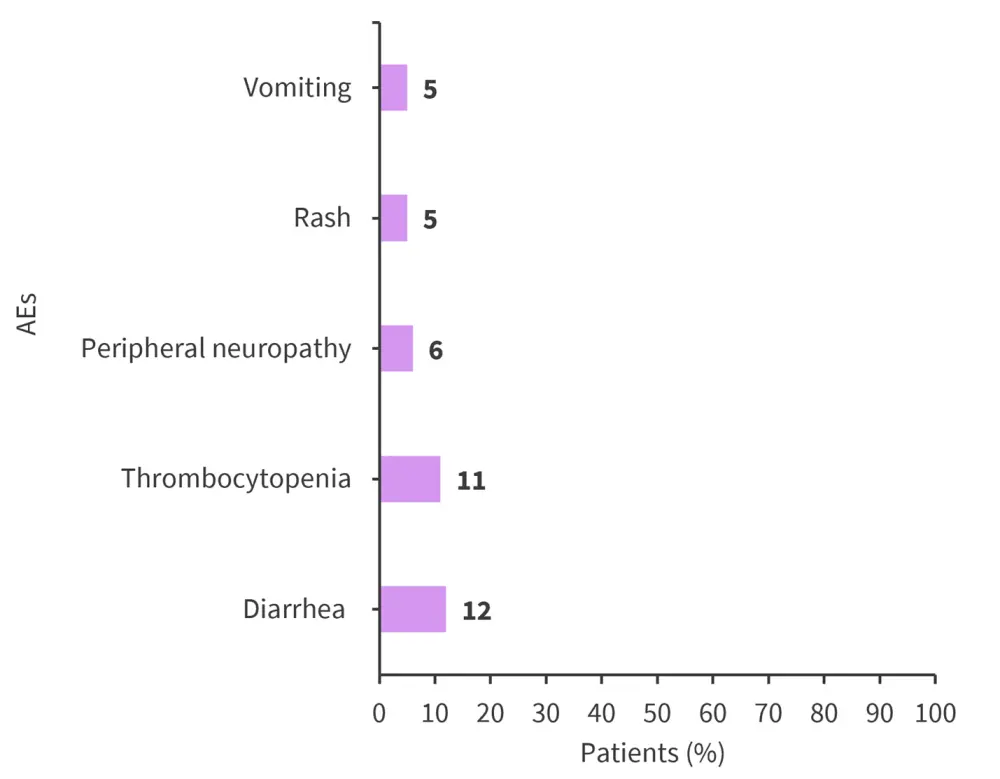
AE, adverse event.
*Adapted from Ludwig et al1
- An Eastern Cooperative Oncology Group performance scale score ≥2 was associated with an increased risk of progression or death (hazard ratio, 2.085)
- A performance scale score ≥2 also reduced the chance of achieving an overall response
- There were no statistically significant differences in safety outcomes
Conclusion
Results from the early access program support the use of ixazomib-based therapy outside of a clinical trial setting. Favorable outcomes were observed in patients with ≥1 and ≥2 prior lines of therapy. Treatment was found to be an effective and well tolerated option for patients diagnosed with RRMM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

