All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
The safety and efficacy of a reduced toxicity regimen in frail patients with NDMM
Your opinion matters
How do you adapt MM treatment regimens for frail patients?
Frail patients diagnosed with multiple myeloma (MM) are not transplant eligible; thus, the recommended frontline treatment consists of a combination of the anti-CD38 antibody, daratumumab, with either lenalidomide and low-dose dexamethasone, or low-dose bortezomib-based combinations. Such regimens appear to be safe and effective for newly diagnosed MM, however, clinical trials have historically included patients of various baseline performance statuses. Therefore, it has been difficult to gauge the precise safety and tolerability in the frail population, which has been reported to achieve shorter survival and higher discontinuation rates.1
Stege et al. recently performed a prospective study; HOVON-143, which explored the efficacy and safety data on a reduced toxicity regimen; ixazomib-daratumumab-low-dose-dexamethasone (Ixa-Dara-dex). This study has been long awaited for being the first to include only frail patients characterized by age or comorbidities. Find below further details on the recently published analysis in the Journal of Clinical Oncology.1 We have previously provided a summary of this and other studies with similar approaches in elderly patients.
Study design
This was a prospective, multicenter phase II trial conducted at 39 hospitals throughout the Netherlands and Belgium. The study design is summarized in Figure 1.
Figure 1. HOVON-143 study design*
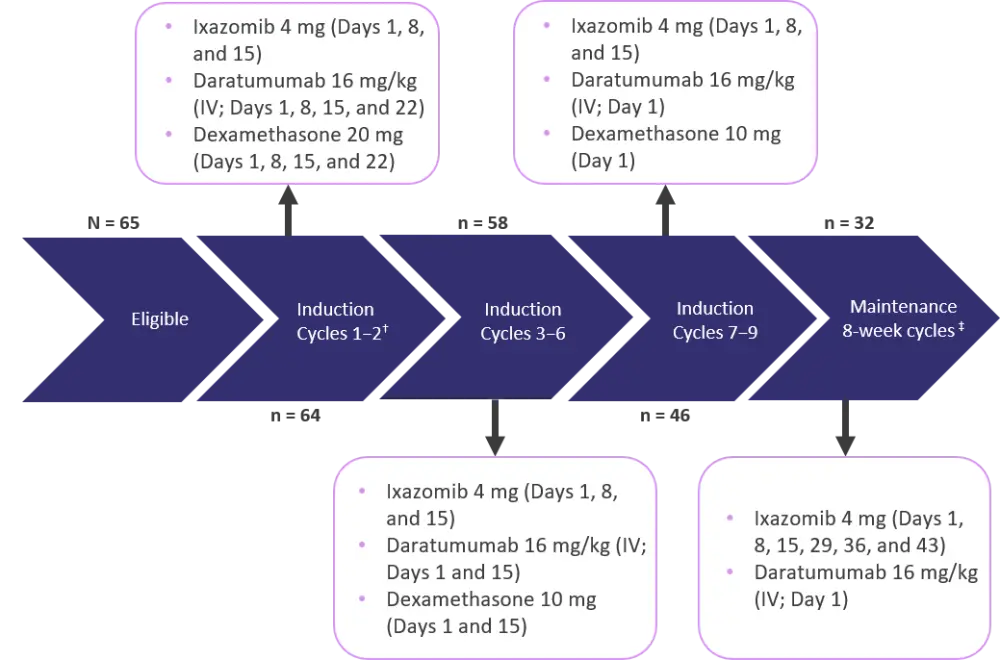
IV, intravenous.
*Adapted from Stege et al.1
ⴕ Each induction cycle (Cycles 1–9) lasted 28 days.
‡Maintenance was performed until progression for a maximum of 2 years.
Eligibility criteria
- Previously untreated symptomatic MM
- Frail patients, according to the IMWG-frailty index
- Absolute neutrophil count ≥1.0 × 109/L; platelet count ≥75 x 109/L
- Must not have severe organ dysfunction (New York Heart Association Class III and IV)
- Creatinine clearance ≥20 mL/min
- Patients with neuropathy Grade ≥1 with pain or Grade ≥2 and active or uncontrolled infections were excluded
Results
Patient characteristics are summarized in Table 1.
Table 1. Patient characteristics*
|
IMWG, International Myeloma Working Group; R-ISS, Revised International Staging System. |
|
|
Characteristic |
N = 65 |
|---|---|
|
Median age, years (range) |
81 (70–92) |
|
Frailty status, % |
|
|
Based on age alone |
20 |
|
Based on other parameters |
49 |
|
Based on both age and other frailty parameters |
31 |
|
IMWG frailty score, %† |
|
|
2 |
49 |
|
3 |
29 |
|
4 |
20 |
|
5 |
2 |
|
R-ISS disease stage, % |
|
|
I |
15 |
|
II |
38 |
|
III |
18 |
|
Unknown |
18 |
|
Median creatinine clearance (mL/min, range) |
56 (20–90) |
Efficacy
The overall response rate was 78% (95% confidence interval, 0.73–0.82), 8% of whom had a stringent complete response, 28% who had a very good partial response, and 43% who had a partial response. The median time to first response was 1 month (range, 1–6 months) and the median duration of response was 11 months (range, 1–26 months). Survival outcomes after a median follow-up of 22.9 months are summarized in Table 2.
Table 2. Survival outcomes after a median follow-up of 22.9 months*
|
CI, confidence interval; OS, overall survival; PFS, progression-free survival. |
||
|
Outcome |
N = 65 |
95% CI |
|---|---|---|
|
Median PFS, months |
13.8 |
|
|
Frail based on age alone |
21.6 |
9.2–not reached |
|
Frail based on other parameters |
13.8 |
7.8–not reached |
|
Both age >80 years and other parameters |
10.1 |
3.3–21.4 |
|
Mortality, % |
38 |
— |
|
12-month OS, % |
78 |
66–87 |
|
Frail based on age alone |
92 |
57–99 |
|
Frail based on other parameters |
78 |
59–89 |
|
Both age >80 years and other parameters |
70 |
44–85 |
A total of 13 patients died while on the treatment protocol, of which three cases were attributed to disease progression. For the other ten patients, causes of non-relapse mortality included infection (40%), organ dysfunction (30%), sudden death (20%), and bleeding because of thrombocytopenia (10%).
Safety
A total of 33 (65%) patients discontinued treatment during induction, with the most common reasons summarized in Table 3.
Table 3. Reasons for discontinuing the ixazomib-daratumumab-low-dose-dexamethasone regimen*
|
*Adapted from Stege et al.1 |
|
|
Reason for discontinuation, % |
N = 65 |
|---|---|
|
Progression |
19 |
|
Intercurrent death |
9 |
|
Toxicity |
9 |
|
Noncompliance to treatment |
6 |
|
Other reasons |
8 |
|
Infection |
6 |
The discontinuation rate was greater in patients aged >80 years with or without frail comorbidities compared with younger patients (30% and 38% vs 13%, respectively).
A total of 32 (49%) patients started maintenance therapy, of whom 47% discontinued after a median follow-up of 13.6 months, mainly because of disease progression.
The incidence of hematologic and non-hematologic Grade ≥3 adverse events and serious adverse events were comparable between the three frail subgroups. The most common events after a median follow-up of 22.9 months are summarized in Table 4.
Table 4. Incidence of Grade ≥3 or serious AEs*
|
AE, adverse event; GI, gastrointestinal. |
|
|
AE, % |
N = 65 |
|---|---|
|
Grade ≥3 hematologic |
31 |
|
Anemia |
3 |
|
Thrombocytopenia |
23 |
|
Neutropenia |
9 |
|
Grade ≥3 non-hematologic |
74 |
|
Infections |
25 |
|
GI |
13 |
|
Cardiac |
11 |
|
Peripheral neuropathy |
6 |
|
Serious AEs |
81.5 |
Finally, all patients completed a health-related quality of life questionnaire at baseline, after three induction cycles (96.6% compliance), and after nine cycles (92.7% compliance). The investigators observed a statistically significant improvement in global health score or quality of life score from baseline to after three cycles, which further improved by cycle nine (54.1, 65.8, and 71.5, respectively; p < 0.001).
Conclusion
Overall, this study demonstrated that a non-toxic regimen could produce effective responses in frail patients. However, patients on this protocol experienced a high rate of Grade ≥3 and serious adverse events, and as such, a relatively high early mortality rate, discontinuation rate, and reduced long-term compliance. Such observations were found both in frail patients characterized by age and by comorbidities. Besides the need to explore alternative regimens with reduced toxicity in this vulnerable population, more trials are necessary with a larger sample size to allow for multivariate analyses and to accurately identify prognostic factors associated with adverse clinical outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

