All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
The GEM-PETHEMA score for assessing the risk of early severe infection
Do you know... The GEM-PETHEMA scoring system assesses the risk of early severe infection. Which variable is included in the novel scoring system?
Patients with multiple myeloma (MM) are highly susceptible to infections, which are associated with high rates of morbidity and mortality.1 Mandatory antibiotic prophylaxis has been used in some studies but remains controversial. While the International Myeloma Working Group has produced recommendations for antibiotic prophylaxis, being able to select the patients that would benefit most from antibiotic treatment would be advantageous.
A study by Encinas et al.1 investigated the creation of a scoring system to identify patients with MM who were at a high risk of developing infections and who may be candidates for antibiotic prophylaxis.
Results
Study Design
To design this scoring system, newly diagnosed patients with MM (N = 1,347) were selected from four Grupo Español de Mieloma (GEM) trials:
- GEM2005 > 65
- GEM2005 < 65
- GEM2010 > 65 (this protocol was later excluded from final analysis as antibiotic prophylaxis was mandatory in the first 3 months)
- GEM2012 < 65
Infectious events were investigated in the first 6 months following treatment. Early severe infections were defined as Grade ≥3 infections, or any grade of pneumonia, in the first 4 months following treatment.
Patient characteristics
Transplant-eligible and -ineligible patients were included in the study; baseline characteristics are shown in Table 1. Most patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0−1, and 57.2% were aged >55 years. High-risk cytogenetics were present in 20.2% of patients, though data were not available for 11.7% (Table 1).
Table 1. Baseline patient characteristics*
|
ECOG PS, Eastern Cooperative Oncology Group performance status; EMD, extramedullary disease; ISS, International Staging System; LDH, lactate dehydrogenase; IgA, Immunoglobulin A; MM, multiple myeloma. |
|
|
Variable |
Percentage of patients |
|---|---|
|
Protocol |
|
|
GEM2005 > 65 |
19.2 |
|
GEM2005 < 65 |
28.9 |
|
GEM2010 > 65 |
17.9 |
|
GEM2012 < 65 |
34.0 |
|
Sex |
|
|
Male |
52.2 |
|
Female |
47.8 |
|
Age, years |
|
|
>75 |
12.5 |
|
≤75 |
87.5 |
|
>55 |
57.2 |
|
≤55 |
42.8 |
|
ECOG PS |
|
|
0−1 |
58.5 |
|
>1 |
41.5 |
|
Hemoglobin (g/dL) |
|
|
≤11 |
56.4 |
|
>11 |
43.6 |
|
ISS stage |
|
|
1−2 |
74.0 |
|
3 |
26.0 |
|
Monoclonal component (g/L) |
|
|
≤40 |
37.8 |
|
>40 |
62.2 |
|
Positive for bone lytic lesions |
86.1 |
|
Positive for EMD |
17.6 |
|
Score FIRST |
|
|
High risk (2 to 5) |
34.5 |
|
Low risk (-3 to 1) |
65.5 |
|
Normal LDH |
84.8 |
|
High-risk cytogenetic abnormalities |
|
|
Yes |
20.2 |
|
Not available |
11.7 |
|
Albumin (g/L) |
19.9 |
|
MM type |
|
|
Non-IgA |
73.6 |
|
IgA |
26.4 |
Infections
During the first 6 months, 24.3% of patients developed ≥1 infection (16.9% developed one infection, 5.5% had two infections, 1.4% had three infections, and 0.52% had >3 infections). At least one severe infection was recorded in 12.5% of patients and, of the patients with any grade of infection, 49.2% were classified as severe.
Respiratory infections were common, as shown in Figure 1, making up 59.1% of total infections. Of the respiratory infections, 21.7% were pneumonia. Overall, fever episodes accounted for 8.7% of the infections recorded and urinary tract infections accounted for 8.2%.
Figure 1. Incidence of any grade and severe infection*
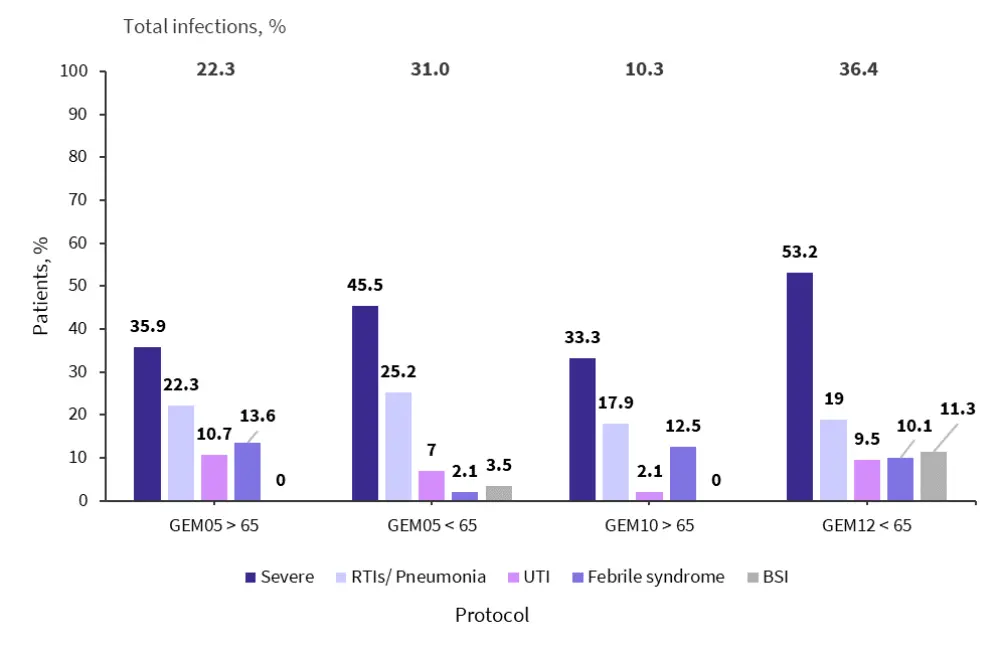
BSI, bloodstream infections; RTI, respiratory tract infection; UTI, urinary tract infection.
*Adapted from Encinas, et al.1
In the first 6 months of treatment, 59 patients died, of which a total of 16 were due to infection in the four trials. If GEM2010 > 65 is excluded, the number who died due to infection falls to 13.
GEM-PETHEMA score development
Following a multivariate analysis of factors associated with an increased risk of severe infection, four variables were selected, as shown in Table 2. The GEM-PETHEMA score was generated by assigning one point to each variable to assess the risk of infection.
Table 2. GEM-PETHEMA score weighting*
|
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; IgA, immunoglobulin A; MM, multiple myeloma. |
||||
|
Variable |
Odds ratio |
p value |
95% CI |
Weight (points) |
|---|---|---|---|---|
|
Albumin ≤30 g/L |
2.12 |
<0.001 |
1.40−3.20 |
1 |
|
ECOG PS >1 |
1.73 |
0.005 |
1.18−2.54 |
1 |
|
Male sex |
1.50 |
0.037 |
1.02−2.20 |
1 |
|
Non-IgA type MM |
1.49 |
0.091 |
0.93−2.39 |
1 |
Using this score, three groups were identified with different probabilities of developing an early severe infection (Table 3). The risk of infection between groups was compared. A comparison of low versus intermediate-risk groups resulted in a probability of early severe infection of 8.2%; low versus high-risk groups gave a probability of 20.6% (sub-hazard ratio, 2.58; 95% CI, 1.80–3.70; p < 0.0001). The score was also tested in the transplant-eligible patients only and produced similar results (area under the curve, 0.62; 95% CI, 0.56–0.67).
Table 3. GEM-PETHEMA risk categories and probability of infection*
|
*Adapted from Encinas, et al.1 |
||
|
Risk category |
Score |
Probability of an early severe infection (%) |
|---|---|---|
|
Low |
0−2 |
8.2 |
|
Intermediate |
3 |
19.2 |
|
High |
4 |
28.3 |
Potential impact of GEM-PETHEMA score in the clinic
The development of this score has the potential to prevent the blanket treatment with antibiotics of all patients with MM. By identifying the patients who are at risk of early, severe, and potentially life-threatening infections, antibiotic treatment can be tailored to the patients that need it most. This reduces the dose of antibiotics required for patients with MM and reduces the risk of inducing antibiotic resistance. Additionally, minimizing unnecessary antibiotic prophylaxis will indirectly help in preserving the intestinal flora.
GEM-PETHEMA score limitations
The clinical trials included in the analysis did not have an incidence of infection as the main endpoint, so the data were extracted during post hoc analysis. The score needs testing in a clinical trial and with real-world patients. The study populations were not homogenous, with both transplant eligible and ineligible patients included in the study.
The GEM2010 > 65 trial was excluded from the final analysis because the protocol used mandatory antibiotic prophylaxis, despite being included in the descriptive analysis. In the other trials included, antibiotic prophylaxis was used at the discretion of the attending physician, but this information was not collected although, in general, antibiotic prophylaxis was not thought to be used.
Patients that underwent autologous hematopoietic stem cell transplant were particularly susceptible to infections in the posttransplant period as a result of the aplasia and mucositis following chemotherapy with melphalan. The impact of comorbidities, immunoparesis, and cumulative steroid dose was not examined.
Anti-CD38 monoclonal antibodies (mAbs) were not used in any of the trials included in this study and can increase the risk of infection for patients. A score has been developed previously that investigates the risk of infection following treatment with mAbs that includes age, lactate dehydrogenase, albumin, and alanine aminotransferase. It is possible that the GEM-PETHEMA score may be updated in the future to incorporate anti-CD338 mAbs if new risk factors emerge.
Conclusion
The aim of the GEM-PETHEMA score is to provide a simple way to stratify a patient’s risk of developing an early severe infection and enable antibiotic prophylaxis to be tailored to those at high risk. The study was able to identify a subset of high-risk patients who had a 20.6% increased risk of infection compared with those in the low-risk group. The GEM-PETHEMA score looks to be a useful tool for physicians, but it would benefit from being tested further in clinical trials and real-world settings. The score may potentially be updated in the future to include treatments such as anti-CD38 mAbs that impact the risk of infection for patients with MM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



