All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
T-cell apheresis for the manufacture of CAR T-cell therapies: Current challenges and future perspectives
Based on data from the KarMMA trial, in March 2021, idecabtagene vicleucel (ide-cel) became the first targeted chimeric antigen receptor (CAR) T-cell therapy for multiple myeloma (MM) approved by the U.S Food and Drug Administration (FDA), which was followed by conditional marketing authorization by the European Commission (EC) in August 2021.
Ide-cel is approved for use after ≥4 prior lines of therapy by the FDA and after ≥3 lines of therapy by the EC; therefore, it has become well-established as a treatment for relapsed/refractory MM (RRMM).
Despite significant amounts of international research and ongoing clinical trials, there are unmet needs in CAR T-cell therapy and challenges, such as its delivery through routine care. As covered recently by the Multiple Myeloma Hub, real-world experience has shown that delays in CAR T-cell therapy production or failure in manufacture can lead to increased morbidity and mortality (available here).
One challenge for CAR T-cell therapy arises from its dependency on apheresis for the collection of the appropriate T-lymphocyte population. Research has focused on maximizing the availability of appropriate T cells for CAR T-cell therapy manufacture. Kunkele et al.1 reported on cryopreserved peripheral blood stem cells (PBSCs) harvested early in the treatment of children with high-risk neuroblastoma (HRNB) for autologous stem cell transplant (ASCT) and stem cell rescue after high-dose chemotherapy. These PBSCs demonstrated viability for CAR T-cell therapy manufacture; whereas apheresis after multiple lines of therapy would carry a higher risk of failure.1 The same viability of PBSCs stored for ASCT, and to be used for later CAR T-cell therapy manufacture, has also been demonstrated in MM.2 Furthermore, research has identified that T cells apheresed for ASCT, which were exposed to granulocyte colony stimulating factor (G-CSF) in patients or in vitro, produced viable CAR T-cell products with no loss of fitness or antitumor activity.3
These findings support the understanding that techniques and approaches to improve and support successful apheresis do exist or are under development. However, current approaches vary significantly between clinical trials and clinical centers, with a lack of accepted and utilized clinical guidelines. Published in the Journal of Clinical Apheresis in December 2021, Thibodeaux et al.4 conducted a search and literature review of National Clinical Trials database (www.clinicaltrials.gov) and performed a qualitative analysis of apheresis techniques described in established CAR T-cell therapeutic clinical trials. Here, the Multiple Myeloma Hub summarizes the results of their work and discusses the articles described above.
CAR T-cell therapy apheresis techniques described in published clinical trials4
Methods
The study by Thibodeaux et al.4 was a qualitative analysis of published clinical trials. The webpage “www.clinicaltrials.gov” was searched using the term “chimeric antigen receptor T cells” on July 1, 2020. Where apheresis was mentioned in the description of the clinical trial, a grounded theory approach was used to identify the subject/context of the mention, allocating this mention to one of 37 context codes, 12 categories, and 4 themes: patient, procedure, product, and miscellaneous.
Results
Of the 670 studies identified, 621 studies were included, with a total of 1,044 mentions of apheresis in 322 studies. Apheresis was mentioned zero times in 299 articles (48%.1), with decreasing numbers of articles featuring increasing mentions of apheresis; only 7 (1.1%) and 5 (0.7%) articles mentioned apheresis 11–15 and 16–20 times, respectively (Figure 1).
Figure 1. Number apheresis mentions in identified articles*
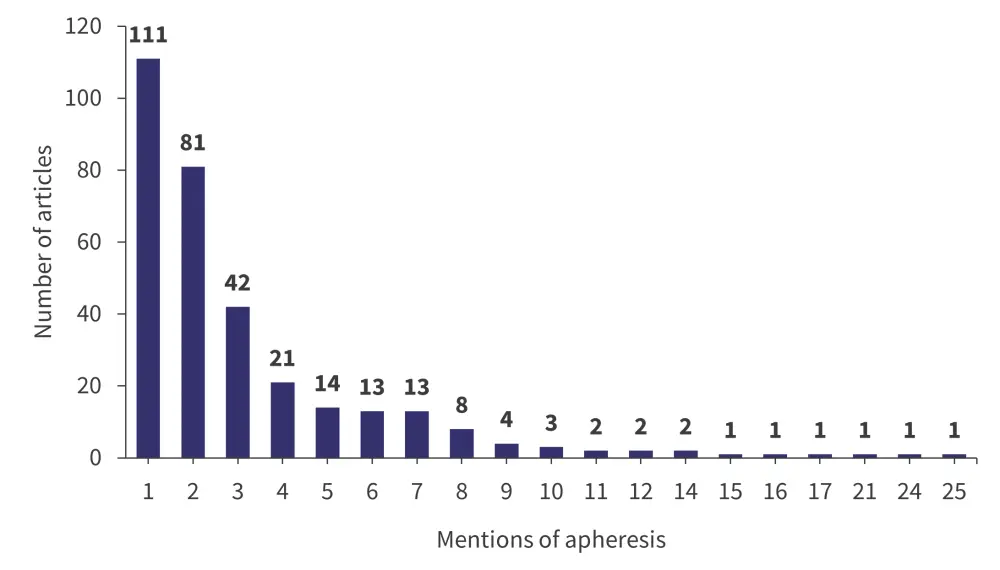
*Data from Thibodeaux, et al.4
Of the studies included in the article, nearly half were phase I studies and ~80% were phase I or phase I/phase II, which demonstrates that the majority were early phase studies (Figure 2).
Figure 2. Distribution of studies according to phase*
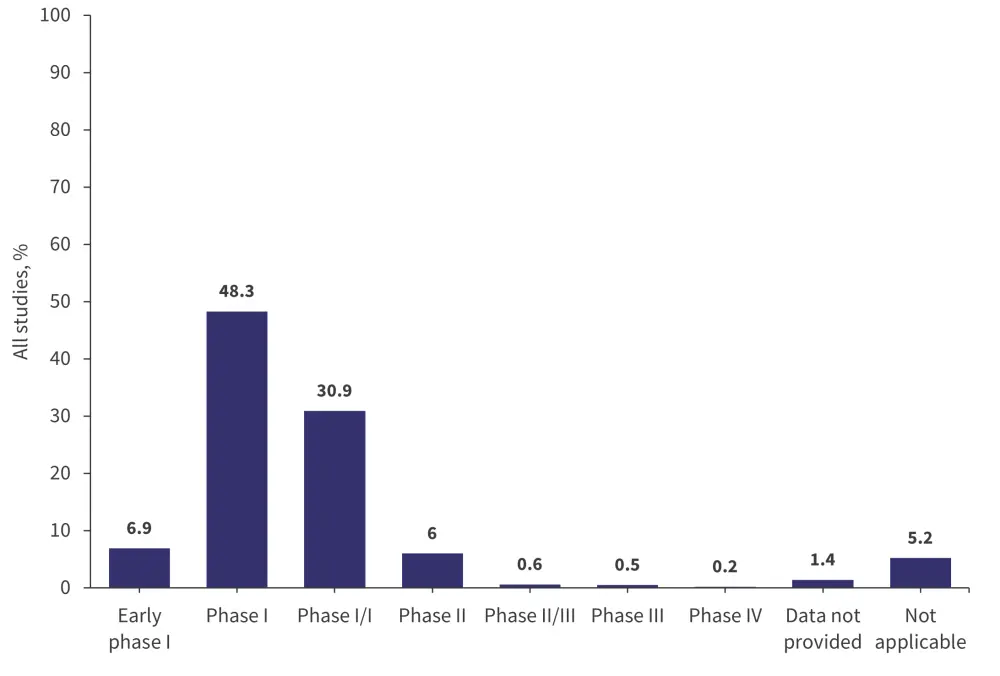
*Data from Thibodeaux, et al.4
In the context of laboratory assessments, platelet count, hemoglobin, and absolute neutrophil count were most commonly mentioned in the context of apheresis, described in only 43.3%, 37.5%, and 35.4% of articles, respectively. Laboratory parameters were reported variably, and with little consistency between the identified articles.
From the thematic analysis, apheresis was mentioned most in the context of peripheral morphonuclear blood cells (22.5%), anticancer therapy within approximately 2 weeks of leukapheresis (15.5%), and patient venous access (8.8%) Of note, only 1.0% and 1.9% of studies mentioned apheresis in the context of product availability and product acceptability, respectively.
Key findings
- The methods used in the collection of CAR T cells are not well reported in clinical study descriptions.
- Nearly 80% of the clinical studies identified were phase I or phase I/II.
- The laboratory parameters used to describe white blood cell, platelet, and red blood cell levels, and transfusion requirements in apheresis were variably reported and described.
- The main themes identified from the study were clinical (e.g., venous access) rather than product-related (e.g., acceptability or availability).
Conclusion
In this literature review, the fact that the majority of clinical trials identified were early phase, means the translation of results into the clinical environment is challenging given a lack of large-scale clinical trials. Reporting on the methods of apheresis used, including the laboratory parameters described, was hugely variable, making validation and reproduction of findings challenging. Challenges in the delivery of CAR T-cell therapies in routine clinical care are likely to differ significantly compared with in early phase clinical trials; therefore, further late phase work is required to identify how these challenges will evolve.
Cryopreserved PBSCs for ASCT in high-risk neuroblastoma1
Early during the course of high-dose chemotherapy, children with HRNB undergo apheresis to collect PBSCs for cryopreservation for subsequent autologous stem cell rescue. In the study by Kunkele at al.1, cryopreserved PBSCs from eight patients with HRNB were evaluated for the presence of defined cell lineages.
Key findings
- CD4 and CD8 precursors were present, with significant numbers of naïve and central memory precursors.
- Large numbers of Ki67+/PD1+ T cells were identified, suggesting cell proliferation following chemotherapy induced lymphopenia.
- Following CD14+ depletion, T cells from G-CSF-exposed PBSCs that had been cryopreserved demonstrated cytokine-driven clonal expansion.
- CD171 CAR+ CD4/CD8 effector cells derived from cryopreserved PBSCs demonstrated anti-neuroblastoma in in vivo murine studies.
Conclusion
The authors conclude that cryopreserved PBSCs represent a viable source for CAR T-cell manufacturing, increasing potential treatment options for patients with high-risk disease, who are likely to have received heavy treatment through multiple lines of therapy.
Cryopreserved PBSCs for the treatment MM2
Krummradt et al.2 conducted a large cohort study in 1,114 patients with MM treated with high-dose chemotherapy and ASCT across several centers in Germany. The study was conducted over a 12-year period, with a minimum follow-up of 6 years.
Key findings
Figure 3. Key findings from a study assessing the collection, storage, and utilization of PBSCs*
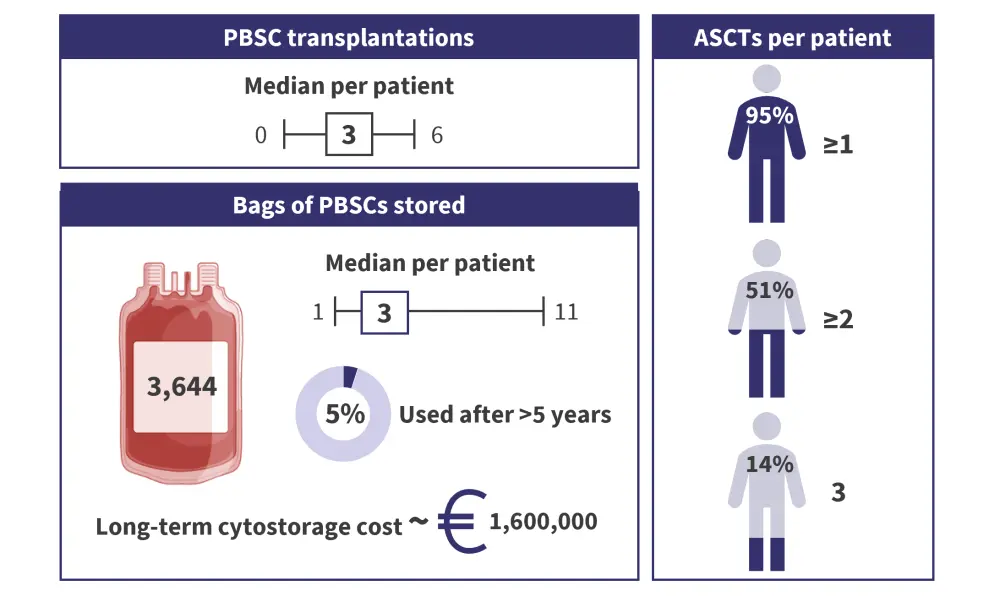
ASCT, autologous stem cell transplantation; PBSC, peripheral blood stem cell.
*Data from Krummradt, et al.2 Created using BioRender.com.
Conclusion
ASCT represents an effective therapy for patients with MM, with most patients receiving up to 2 or 3 transplants. The amount of PBSCs generated can vary significantly, affecting treatment decisions and storage considerations. Only a small percentage of cryopreserved PBSCs are utilized, increasing storage costs and raising ethical and practical concerns over further storage or disposal.
Efficacy of T cells isolated from G-CSF-treated patients with MM for CAR T-cell therapy manufacture3
Using the academic B-cell maturation antigen-targeted CAR T-cell therapy, ARI0002h (ARI2h; currently under investigation in the phase I clinical trial NCT04309981), Battram et al.3 studied whether G-CSF treatment of freshly isolated T cells from patients with MM affected their potential to be used for the manufacture of effective CAR T cells. Both in vitro (i.e., cell culture) and in vivo laboratory studies were conducted.
Key findings
- Treatment of patients with G-CSF treatment did not alter the CD4:CD8 T cell ratio, increase the proportion of regulatory T cells, or lead to T-cell dysfunction.
- G-CSF had no impact on ARI2h expression or activity in vitro (G-CSF-exposed cell culture).
- G-CSF reduced the number of stem-cell memory-like T cells, but not any other effector or memory T-cell populations in vivo.
- Pre and post G-CSF treatment, ARI2h cells demonstrated equal antitumor activity in both in vitro and in vivo (murine models).
- Immunohistochemical staining of ARI2h cells before and after G-CSF exposure demonstrated no significant difference in the cell surface phenotype of CAR T cells.
Conclusion
The authors conclude that whether administered to patients, or applied to cell culture, G-CSF has a negligible effect on T-cell phenotype when added in vitro or administered to patients. CAR T-cell fitness and efficacy are unaffected when produced from G-CSF-exposed progenitor cells, and cells produced through ASCT apheresis are a viable source of T cells for CAR T-cell manufacture in MM.
Discussion
Apheresis is essential to generate T lymphocytes for the manufacture of CAR T cells. Recent clinical trials do not comprehensively describe current laboratory parameters or clinical techniques/methods, which confounds meta-analysis and the determination of optimal approaches. In addition, there are no widely accepted and utilized clinical guidelines on apheresis. New approaches, such as the use of G-CSF and cryopreserved PBSCs collected for ASCT, may help to increase the availability of CAR T-cell therapies for the treatment of MM, without compromising quality or safety.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



