All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Efficacy and safety of ARI0002h, an academic BCMA-directed CAR T-cell therapy for RRMM
Despite significant improvements in multiple myeloma (MM) survival in the last year, most patients will relapse and progress, developing relapsed/refractory (RR)MM.1 RRMM that is refractory to the main three groups of treatment agents has a poor prognosis, no clear treatment pathway, and a very poor overall survival. A potential therapeutic option for these patients is immunotherapy using chimeric antigen receptor T-cells (CAR T-cells). In addition, B-cell maturation antigen (BCMA) expression is restricted to plasma cells, making it an optimal target for immunotherapy in MM.
Two CAR T-cell products are currently approved for MM treatment in Europe; idecabtagene vicleucel (our summary of the KarMMa study and the European Commission approval can be read here) and ciltacabtagene autoleucel (our summary of the conditional European Commission approval can be viewed here), which have both demonstrated promising results in heavily treated patients. At the European Hematology Association (EHA) 2022 Congress, Carlos Fernández de Larrea gave a presentation on the CARTBCMA-HCB-01 clinical trial (NCT04309981), which investigated ARI0002h, a CAR T-cell therapy that was developed entirely within an academic institution.1
ARI0002h is a 4-1BB based CAR T-cell product containing a humanized single chain variable BCMA-targeting fragment with proven in vitro and in vivo efficacy.2 In the CARTBCMA-HCB-01 trial, five Spanish centers were included, two of which were involved in ARI0002h production.1
Study design and patient characteristics1
The study design of CARTBCMA-HCB-01 is depicted in Figure 1. Fractionating the infusion of ARI0002h had previously been found to reduce side effects. Initially, there were 44 patients assessed for eligibility and 35 screened. A total of 30 were allocated to the intervention, and five of these patients progressed or died prior to the first infusion with ARI0002h. Following apheresis, 50% of patients required bridging therapy.
Figure 1. Study design*
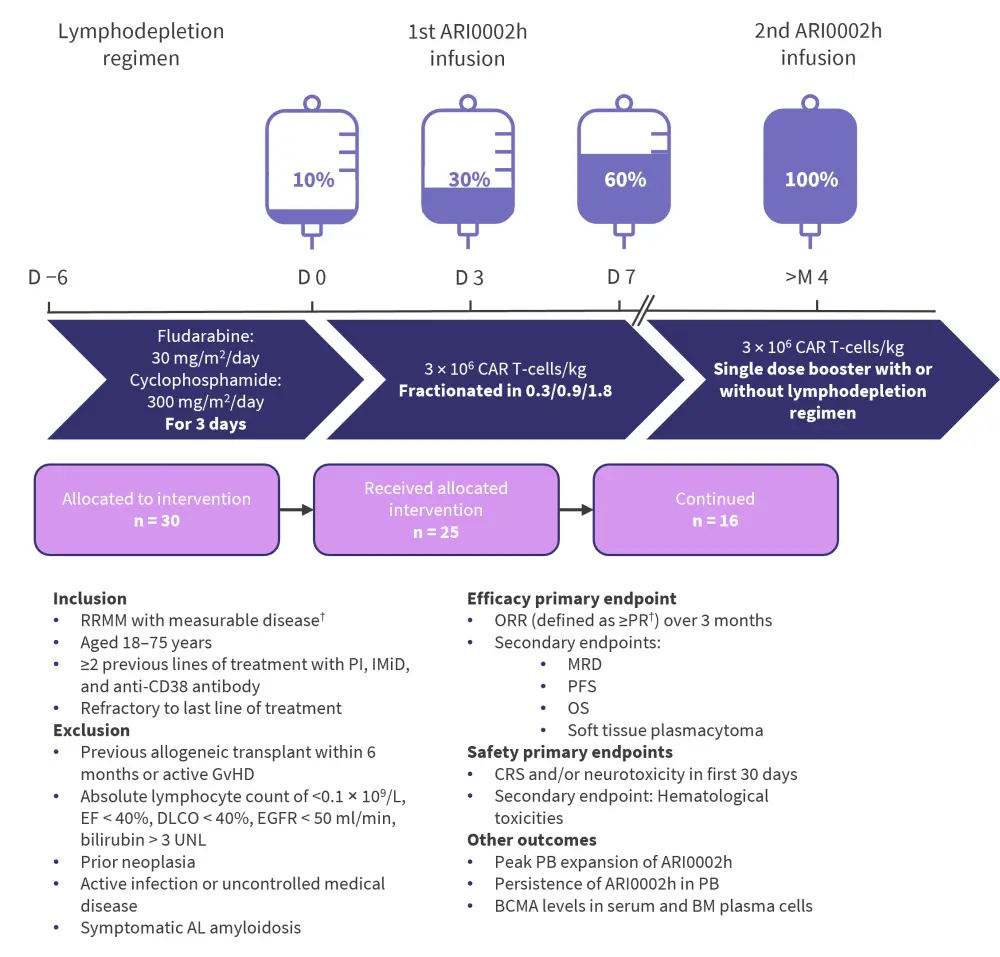
BCMA, B-cell maturation antigen; BM, bone marrow; CAR T-cell, chimeric antigen receptor T-cell; CRS, cytokine release syndrome; D, day; DLCO, carbon monoxide diffusion capacity; EF, ejection fraction; EGFR, epidermal growth factor receptor; GvHD, graft-versus-host disease; IMiD, immunomodulatory drug; M, month; MRD, minimal residual disease; ORR, overall response rate; OS, overall survival; PB, peripheral blood; PFS, progression-free survival; PI, proteasome inhibitor; PR, partial response; RRMM, relapsed/refractory multiple myeloma; UNL, upper normal limit.
*Adapted from Fernández de Larrea.1
†International Myeloma Working Group.
All patients included in the study had been triple exposed to a proteasome inhibitor, an immunomodulatory drug, and to anti-CD38 antibody treatment, and most patients (87%) had received a prior autologous stem cell transplant (Table 1). The median manufacturing time of ARI0002h was 11 days (range, 9–14 days).
Table 1. Patient characteristics*
|
*Adapted from Fernández de Larrea.1 |
|
|
Characteristic, % (unless otherwise stated) |
N = 30 |
|---|---|
|
Median age (range), years |
61 (36–74) |
|
Sex |
|
|
Female |
40 |
|
Male |
60 |
|
Heavy chain isotope |
|
|
IgG |
47 |
|
IgA |
23 |
|
Bence Jones |
23 |
|
Plasmacytomas |
47 |
|
Extramedullary |
20 |
|
High-risk cytogenetics† |
33 |
|
Median plasma cells in bone marrow (range), n |
11 (0–100) |
|
Median number of previous lines of therapy (range), n |
4 (2–10) |
|
Prior autologous stem cell transplantation |
87 |
|
Prior allogeneic stem cell transplantation |
13 |
|
Documented triple-exposed‡ |
100 |
|
Documented triple-refractory‡ |
67 |
Results1
Efficacy
The overall response rate (≥ partial response) following the first infusion and at a median follow-up of 17.5 months was 100% (Figure 2), with 63% of patients achieving a stringent complete response.
Figure 2. Response rates in patients treated with ARI0002h*
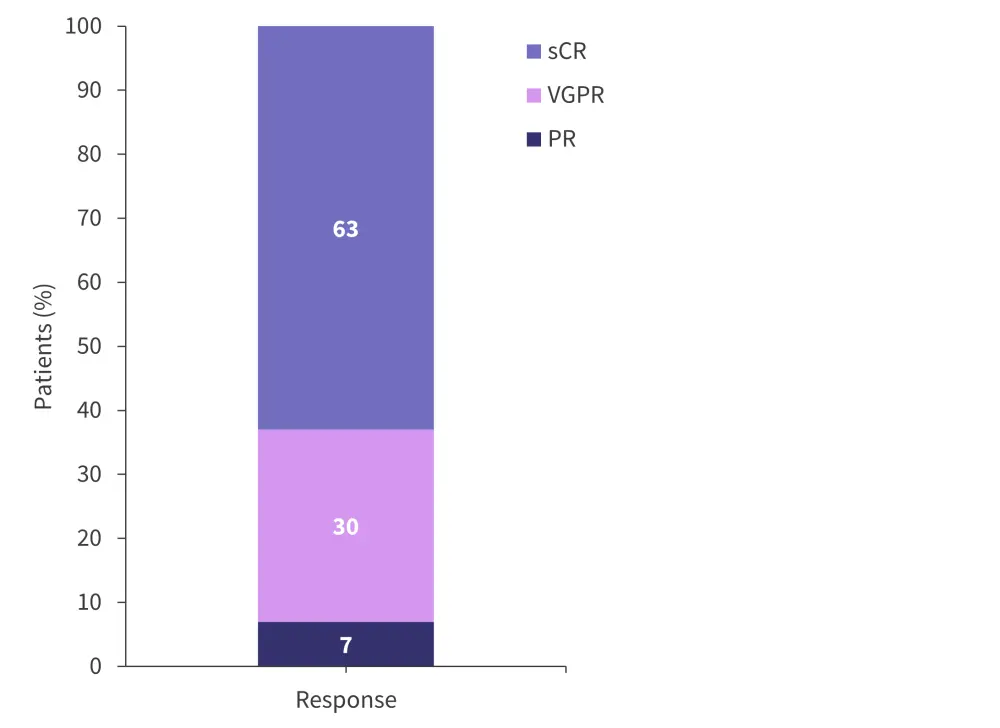
PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Fernández de Larrea.1
Minimal residual disease negativity was achieved in 70% of patients 28 days following the first infusion and in 53% of patients after 12 months (Table 2). Median progression-free survival and overall survival were not reached during the median 17.5 month follow-up period.
Table 2. Secondary efficacy outcomes*
|
CR, complete response; MRD, minimal residual disease; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; VGPR, very good partial response. |
|
|
Secondary outcome, % |
|
|---|---|
|
MRD negativity† in ITT population |
|
|
Day +28 |
70 |
|
Day +100 |
80 |
|
Month 6 |
67 |
|
Month 12 |
53 |
|
PFS at 18 months |
53 |
|
OS at 18 months |
73 |
|
ORR of patients with soft tissue plasmacytoma at Day 100‡ |
93 |
|
CR |
57 |
|
VGPR |
29 |
|
PR |
7 |
|
Progressive disease |
7 |
A total of 24 patients went on to have a second “booster” infusion, of which 58% were in stringent complete response at the time of their reinfusion. Of those reinfused, 50% of patients had peripheral blood CAR T-cell expansion, 25% had an improved response, and 17% maintained their response.
Safety and other outcomes
Cytokine release syndrome (CRS) was noted in 90% of patients, which was mostly Grade 1 (67%), and there were no neurotoxic adverse events observed (Table 3). All patients presented with cytopenias beyond 30 days following infusion, and there were three cases of macrophage-activation syndrome, one case of hepatitis-B activation, and one case of colon cancer that was considered unrelated to ARI0002h infusion. There were no CRS or neurotoxic adverse events after the second infusion.
Table 3. Adverse events following infusion with ARI0002h*
|
CRS, cytokine release syndrome. |
|
|
Adverse event, % (unless otherwise stated) |
|
|---|---|
|
CRS |
90 |
|
Grade 1 |
67 |
|
Grade 2 |
23 |
|
≥Grade 3 |
0 |
|
Median onset of CRS (range), days |
8 (1–10) |
|
Median duration of CRS (range), days |
4 (1–12) |
|
Use of tocilizumab for CRS† |
74 |
|
Use of corticosteroids |
10 |
|
Neurotoxicity |
0 |
|
Hematological toxicity, all (Grade 3/4) |
|
|
Thrombocytopenia |
100 (100) |
|
Neutropenia |
87 (69) |
|
Anemia |
90 (52) |
|
Median duration of hematological toxicity (range), months |
|
|
Thrombocytopenia |
4 (0–14) |
|
Neutropenia |
9 (1–19) |
|
Anemia |
5 (0–20) |
CAR T-cells were detected in peripheral blood in 62%, 36%, and 20% of patients at Day 100, 6 months, and 12 months, respectively. The median maximum peripheral blood expansion was 14 days (range, 7 days to 6 months) and the median persistence was 5 months (range, 2 months to not reached). Of the patients with available samples, 33% who relapsed still had detectable CAR T-cells in peripheral blood.
Serum BCMA expression was significantly reduced 3 months after infusion (p = 0.0007), with increasing levels associated with relapse. BCMA expression in bone marrow plasma cells at the end of treatment was significantly lower in patients who relapsed compared to baseline expression in all patients (p = 0.0053).
Conclusion
Fernández de Larrea concluded that production of ARI0002h was fast and feasible in patients with RRMM and that infusion resulted in deep and sustained responses with relatively low-grade toxicity in the form of Grade 1 and 2 CRS and no neurotoxic adverse events. Reinfusion was possible in most patients, was not associated with any toxicity, and improved response rates in some patients.
Typically, approved CAR T-cell products have to be ordered from the licensed pharmaceutical company, with manufacturing being subject to capability according to demand.3 This, along with cost, has the potential to result in bottlenecks in the production of CAR T-cell products, which ultimately delay treatment for patients with relapsing disease. As ARI0002h is produced entirely in an academic institution, manufacturing time is low and less likely to be subject to the hold-ups that may be associated with other CAR T-cell products.
Another presentation at the EHA2022 Congress discussed a different academically produced CAR T‑cell product (HSP-CAR30) for patients with Hodgkin lymphoma and CD30+ T-cell lymphoma.4 This phase I study (NCT04653649) of 10 patients demonstrated promising results with an overall response rate of 100% and a CR rate of 50%. The safety profile was favorable, with CRS experienced in 60% of patients (all Grade 1), no neurotoxicity, skin rashes in 40% of patients, and long-lasting cytopenias in only 20% of patients. Both studies1,4 suggest that there is an increasing appetite for academically produced CAR T-cell products that enable rapid access to these therapeutics.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



