All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Ide-cel in RRMM: Real-world experience and biomarkers of response
Do you know... Which of the following is an independent predictor of inferior progression-free survival in patients receiving ide-cel?
Idecabtagene vicleucel (Ide-cel) was approved by the U.S. Food and Drug Administration (FDA) in March 2021 for the treatment of patients with relapsed/refractory multiple myeloma (RRMM) who have received at least four previous lines of therapy.
More than 12 months later, real-world experience in the delivery of chimeric antigen receptor (CAR) T-cell treatments suggest that delays in their production and delivery cause increased morbidity and mortality in patients with RRMM who are on waiting lists.
Identification of diagnostic biomarkers and patient characteristics early in the CAR T-cell treatment process (e.g., before apheresis and CAR T-cell therapy production) that are associated with clinical outcomes and response to treatment can be useful for the prognostication of patients and facilitate treatment decisions.
The Multiple Myeloma Hub previously published an article and an expert opinion on the clinical outcomes of ide-cel in the KarMMA trial (NCT03361748). During the 19th International Myeloma Society (IMS) Annual Meeting, Doris Hansen presented real-world data on patients with RRMM treated with ide-cel,1 which we summarize here. In addition, we discuss data published by Rytlewski, et al.2 at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting from a real-world analysis defining how patient profiles correlate with manufacturing and clinical endpoints for patients with RRMM treated with ide-cel.
Ide-cel for RRMM in clinical practice1
Study design
The study presented by Hansen1 was a multicenter observational study of patients receiving ide-cel infusion at 11 centers across the US. Data from the ide-cel cohort (referred to as the real-world cohort) were compared with data from the KarMMa trial. Baseline characteristics of these two cohorts are presented in Table 1. Univariate and multivariate analyses, including previous B‑cell maturation antigen (BCMA)-targeted therapy, high-risk cytogenetics, extramedullary disease, Eastern Cooperative Oncology Group performance status (ECOG PS), penta-refractory status, ide-cel dose, and patient age, were performed.
Table 1. Baseline characteristics of the real-world and KarMMa cohorts*
|
ECOG, Eastern Cooperative Oncology Group; PS, performance status; R-ISS, Revised International Staging System. |
||
|
Characteristic, % (unless stated otherwise) |
Real-world cohort |
KarMMa cohort |
|---|---|---|
|
Median age, years |
64 |
61 |
|
Male |
58 |
59 |
|
ECOG PS |
|
|
|
0–1 |
82 |
98 |
|
2–4 |
20 |
2 |
|
R-ISS |
|
|
|
I |
18 |
11 |
|
II |
54 |
70 |
|
III |
28 |
16 |
|
Extramedullary disease |
47 |
39 |
|
High-risk cytogenetics |
38 |
35 |
|
Autologous transplant |
84 |
94 |
|
Median prior lines of therapy, n |
7 |
6 |
|
Penta-refractory disease |
41 |
26 |
Results
Of 196 patients in the real-world cohort, 159 patients were infused, 20 were awaiting infusions, 12 had died due to disease progression, and 5 experienced manufacturing failure. In total, 147 patients were evaluable for overall response rate and 159 patients were evaluable for progression-free survival (PFS) and overall survival (OS). The median time to CAR T-cell therapy infusion was 47 days.
Overall, 77% of patients from the real-world cohort would not have been eligible for the KaRMMa trial due to renal insufficiency, previous exposure to prior BCMA-directed therapy, cytopenias, or an ECOG PS of 2–4.
Responses at Day 30, Day 90, and overall response rate were evaluated.
- In total, 40% of patients in the real-world cohort achieved complete response (CR) or stringent CR as their best overall response, as shown in Figure 1.
- Median PFS was 8.9 months (95% confidence interval [CI], 8.5 to not reached; median follow-up, 5.3 months) in the real-world cohort, compared with 8.8 months (95% CI, 5.6–11.6 months; median follow-up, 13.3 months) in the KaRMMa cohort.
- The real-world cohort revealed a 6-month OS estimate of 84% (95% CI, 77–91 and the KaRMMa cohort reported a median OS of 19.4 months.
Figure 1. Response rates in the real-world cohort*
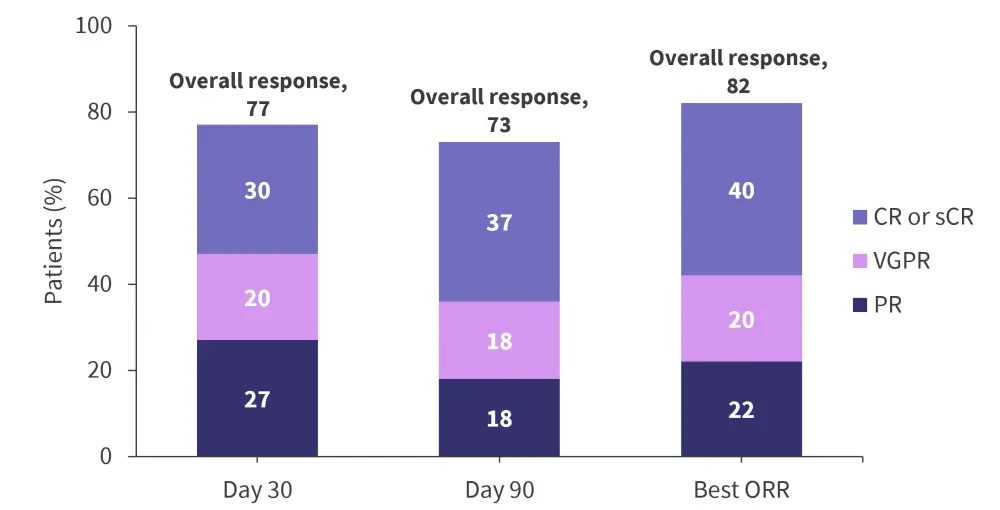
CR, completed response; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Hansen.
Multivariate analysis
In the multivariate analysis, for the real-word cohort, prior anti-BCMA therapy was predictive of an inferior best response ≥CR (odds ratio [OR], 0.28; 95% CI, 0.09–0.84; p = 0.03) and PFS (hazard ratio [HR], 3.20; 95% CI, 1.35–7.61; p = 0.008), and high risk cytogenetics was predictive of inferior PFS (HR, 2.50; 95% CI, 1.22–5.13; p = 0.012).
Safety
-
- In total, 21 deaths were reported at the data cut-off, 13 due to myeloma progression, 3 due to toxicity, 2 due to hemophagocytic lymphohistiocytosis, 3 due to COVID-19, and 1 due to cardiomyopathy.
- Common adverse events included cytokine release syndrome and neurotoxicity (Figure 2).
- Grade ≥3 cytokine release syndrome occurred in 3% of patients and was more likely in patients with ECOG PS ≥2 (p = 0.004), R-ISS Stage III (p = 0.024), or high marrow disease burden (p = 0.046).
- Grade ≥2 neurotoxicity occurred in 11% of patients and was more likely in patients with ECOG PS ≥2 (p = 0.004), elevated baseline ferritin (p = 0.010), or β2-microglobulin >5.5 mg/L (p = 0.001), or those who received bridging chemotherapy (p=0.014) or a CAR T-cell dose ≥400 x 106 (p = 0.036)
Figure 2. Safety in the real-world and KarMMa cohorts*
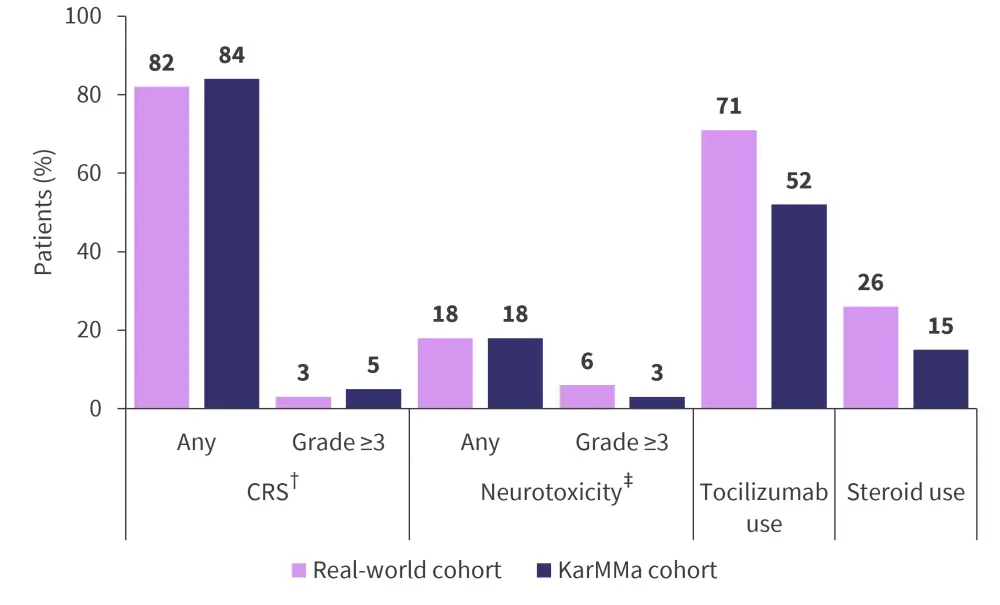
ASTCT, American Society for Transplantation and Cellular Therapy; CRS, cytokine release syndrome.
*Adapted from Hansen.1
†The ASTCT criteria were used for grading CRS.
‡The ASTCT criteria were used for grading neurotoxicity.
Overall, this retrospective study showed promising real-world outcomes of ide-cel in patients with RRMM, and it is important to recognize that many of the real-world patients would not have been eligible for the KaRMMa trial. Further research is required to understand the outcomes based on the timing, duration, and mechanism of action of BCMA-directed therapy.
Patient profiles are associated with good clinical outcomes with ide-cel treatment in clinical trials2
Study design
The study published by Rytlewski, et al.2 was a retrospective, multivariate analysis of 164 patients with RRMM treated with ide-cel in the KaRMMa trial (NCT03361748) and Cohort 1 of the KarMMa-2 trial (NCT03601078). The analysis included 112 clinical variables, 19 manufacturing variables, 50 peripheral blood mononuclear cell (PBMC) variables, 19 manufacturing valuables, and 6 clinical endpoints.
The primary objective was to identify patient profiles that were correlated with durable outcomes in patients treated with ide-cel using machine learning.
Results
The study used PBMC, process, and product variables to define four patient subgroups or clusters. These clusters showed significantly different clinical outcomes according to PFS and CRR, suggesting a connection between poor manufacturing profiles and less durable clinical efficacy. Cluster 4 demonstrated the best clinical outcomes and cluster 1 presented the least favorable outcomes. Cluster 2 contained the most patients and was associated with intermediate manufacturing endpoints (Figure 3).
Cluster 4 was the most favorable and, compared to cluster 1, was characterized by the following:
-
-
- a 2-fold higher proportion of CD3+ cells in PBMCs;
- a better CD4:CD8 ratio;
- a 25% higher Day 5 cell size;
- a higher potency, vector copy number, and proportion of cells expressing CAR; and
- a 3-fold higher CAR T-cell yield.
-
Figure 3. Patient and manufacturing variables considered in clusters
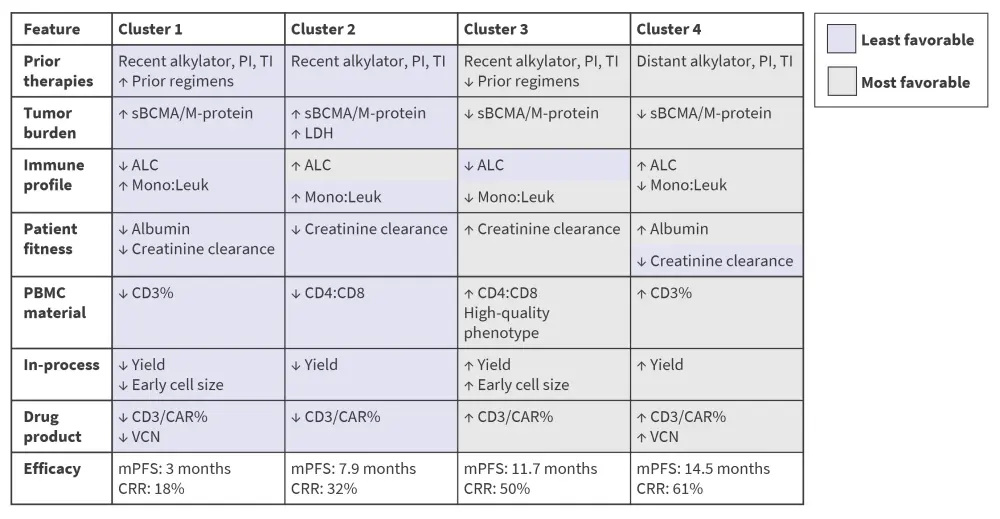
ALC, absolute lymphocyte count; CAR, chimeric antigen receptor; CRR, complete remission rate; Leuk, leukocyte; Mono, monocyte; mPFS, median progression-free survival; PI, protease inhibitor; TI, transfusion independence; sBCMA, serum B-cell maturation antigen; VCN, vector copy number.
*Adapted from Rytlewski, et al.2
The study identified six clinical parameters at the stage of patient screening for CAR T-cell therapy, which influenced treatment response, as the least favorable and most favorable influencing factors (Table 2).
Table 2. Clinical screening factors influencing outcomes of CAR T-cell treatment*
|
B2M, β2-microglobulin; CAR, chimeric antigen receptor. |
||
|
Patient characteristic |
Least favorable |
Most favorable |
|---|---|---|
|
Platelets, 109/L |
130 |
190 |
|
B2M, 109/L |
4 |
2 |
|
Lymphocyte count, 109/L |
0.5 |
0.9 |
|
Albumin, 109/L |
35 |
40 |
|
M-protein, 109/L |
15 |
5 |
|
Time from previous alkylator, months |
≤6 |
>6 |
Safety
Cytokine release syndrome and neurological toxicities were present in all four clusters (Figure 4).
Figure 4. Adverse events*
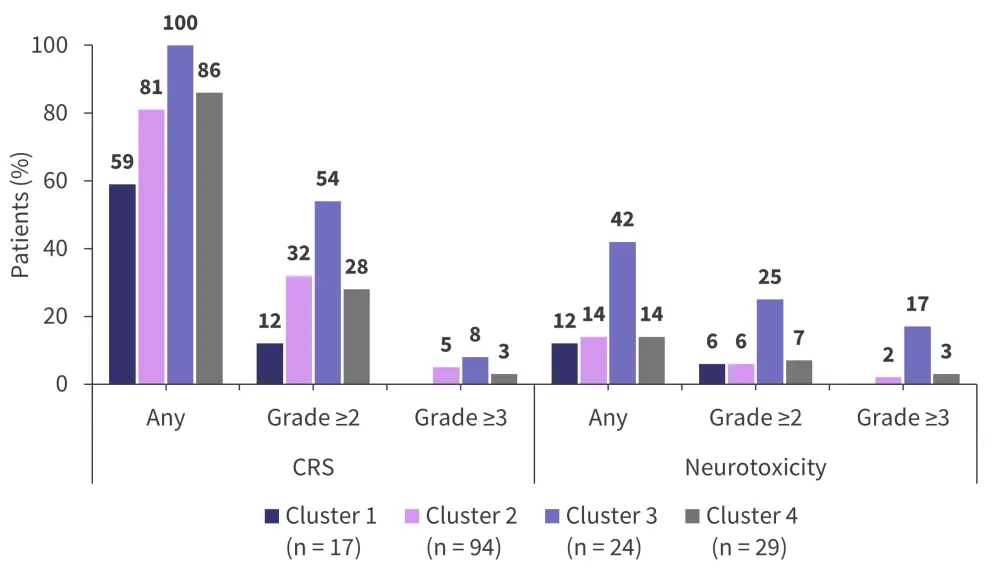
CRS, cytokine release syndrome.
*Adapted from Rytlewski, et al.2
Overall, the study shown a good correlation between laboratory parameters and longitudinal outcomes in patients with RRMM.
Conclusion
Real-world evidence supports the favorable outcomes of ide-cel or T-cell therapy for patients with RRMM, with promising correlations between clinical parameters and the longitudinal outcomes. Further work is needed to identify biomarkers early in the disease course of patients with RRMM that are reflective of an effective manufacturing process and indicative of good response to CAR T-cell treatment and correlate with favorable clinical outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




