All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Do you know... Which group of patients with RRMM are teclistamab, elranatamab, and talquetamab indicated for?
Video series
During the Multiple Myeloma Hub virtual symposium held on March 11, 2024, “Current and future perspectives for bispecific antibodies in multiple myeloma: Learnings from 2023,” Naresh Bumma, The Ohio State University, Columbus, US, delivered a presentation on B-cell maturation antigen (BCMA)-directed bispecific antibodies in multiple myeloma (MM).
Bumma provided an overview of T-cell engagement in relapsed/ refractory MM (Figure 1), sharing the latest clinical trial data on single agents and combination therapies in MM, including BCMA-directed bispecific antibodies (Figure 2). Bumma highlighted patient-specific clinical factors, preferences, and access as determinants of treatment selection, then closed with an overview of strategies to effectively manage adverse events and infections associated with the use of bispecific antibodies.
Figure 1. T-cell engagement in RRMM*
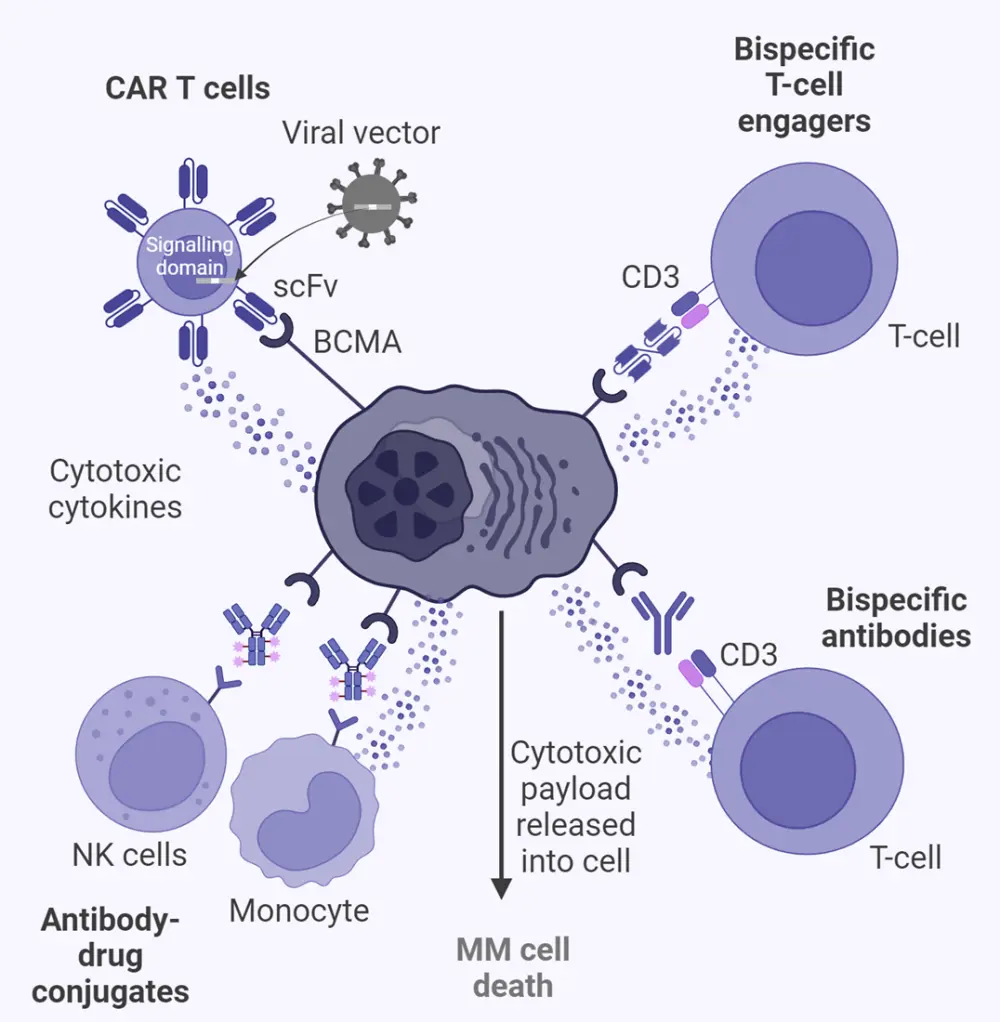
Ab, antibody; BCMA, B-cell maturation antigen; BM, bone marrow; CAR, chimeric antigen receptor; FcRH5, Fc receptor-homolog 5; GC, germinal center; GPRC5D, G protein–coupled receptor class C group 5 member D; IFN, Interferon; IL, interleukin; MM, multiple myeloma; NK, natural killer; PC, plasma cell; RR, relapsed/refractory; scFv, single-chain fragment variable; SLAM, signaling lymphocytic activation molecule; TNF, tumor necrosis factor.
*Adapted from Cho, et al.1
Figure 2. Summary of patient characteristics and topline data from clinical trials of bispecific antibody combinations*
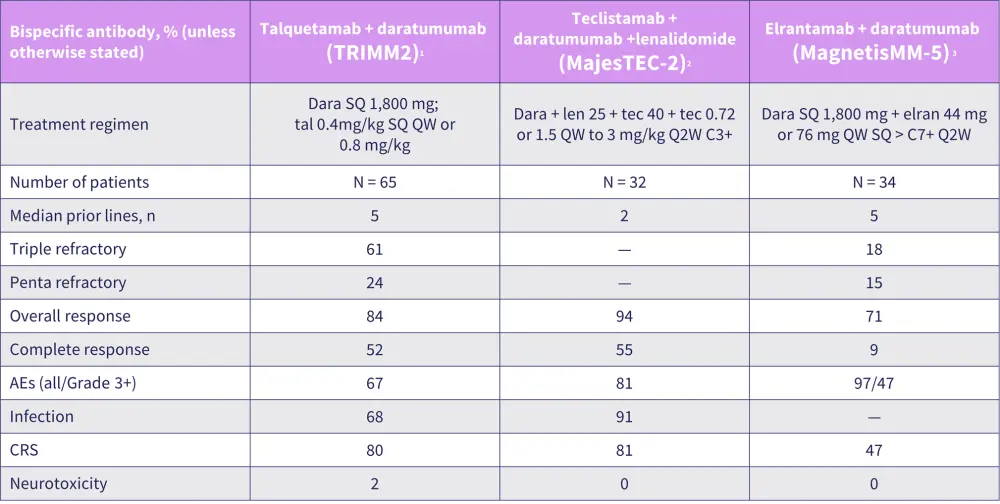
AE, adverse event; C, cycle; CRS, cytokine release syndrome; dara, daratumumab; elran, elranatamab; len, lenalidomide; SC, subcutaneous; tec, teclistamab; QW, every week; Q2W, biweekly.
*Data from Dholaria, et al.2; Searle, et al.3; Grosicki, et al.4
This independent medical activity was funded by Janssen and Bristol Myers Squibb. All content was developed independently by the faculty. The funders were allowed no influence on the content of this activity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Naresh Bumma
Naresh Bumma



