All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Survival rates and impact of MRD using MFC and NGS in newly diagnosed multiple myeloma patients – results from the FORTE trial
The 62nd American Society of Hematology (ASH) Annual Meeting and Exposition was held in December 2020. Findings of the survival analysis from the FORTE trial (NCT02203643) were presented in two separate oral abstracts by Francesca Gay and Stefania Oliva.1,2
Study design
The FORTE trial was a two-staged randomized study that enrolled patients with newly diagnosed multiple myeloma (NDMM), ≤ 65 years old, and eligible for transplantation. Patients were first randomized to one of the three arms as detailed in Figure 1. The drug doses were the following:
- Carfilzomib (K): 36 mg/m2 on days 1–2, 8–9, and 15–16
- Cyclophosphamide (C): 300 mg/m2 on days 1, 8, and 15
- Lenalidomide (R): 25 mg on days 1–21
- Dexamethasone (d): 20 mg on days 1–2, 8–9, 15–16, and 22–23
The carfilzomib plus cyclophosphamide-dexamethasone (KCd) and carfilzomib plus lenalidomide-dexamethasone (KRd) groups were given a single autologous stem cell transplant (ASCT) following four induction cycles. For the second randomization, patients were either randomized to lenalidomide or carfilzomib and lenalidomide maintenance. The maintenance phase drug doses were as follows:
- K: 36 mg/m2 on days 1, 2, 15, and 16 for up to 2 years
- R: 10 mg on days 1–21 until progression or intolerance
Figure 1. Trial design1,2
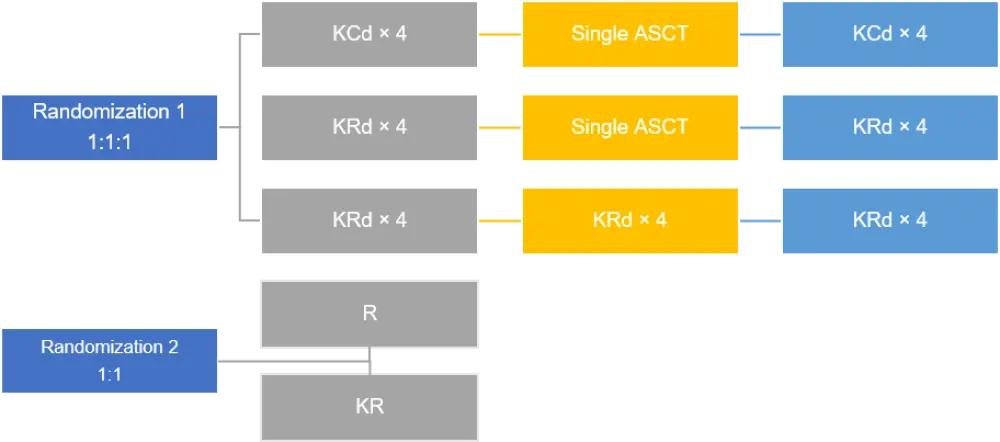
ASCT, autologous stem cell transplant; C, cyclophosphamide; d, dexamethasone; HDM, high-dose melphalan; K, carfilzomib; R, lenalidomide.
Findings from the premaintenance and subgroup analysis in the intention-to-treat (ITT) population have been previously reported here.
Results
Survival and safety analysis – updated at ASH 2020 (Aim 1)
Francesca Gay et al1 evaluated progression-free survival (PFS) of KRd induction-ASCT-KRd consolidation (KRd_ASCT) vs 12 cycles of KRd (KRd12) vs KCd induction-ASCT-KCd consolidation (KCd_ASCT), and the PFS of KR vs R maintenance regime.
Patient baseline characteristics were well balanced in all three arms for Randomization 1 (R1) and two arms for Randomization 2 (R2); see Table 1.
Median follow-up at R1 was 45 months and at R2 was 31 months.
Table 1. Patient characteristics1
|
ASCT, autologous stem cell transplant; C, cyclophosphamide; d, dexamethasone; FISH, fluorescence in situ hybridization; ISS, International Staging System; K, carfilzomib; R, lenalidomide; R-ISS, Revised International Staging System. *Calculated on the available patients. |
|||||
|
Baseline characteristic |
Randomization 1 |
Randomization 2 |
|||
|---|---|---|---|---|---|
|
KCd_ASCT |
KRd_ASCT |
KRd12 |
KR |
R |
|
|
Median age (range) |
57 (52–62) |
57 (52–62) |
57 (51–62) |
56 (52–62) |
57 (51–62) |
|
≥ 60 years (%) |
62 (39) |
62 (39) |
60 (38) |
62 (35) |
66 (37) |
|
ISS stage III, n (%) |
28 (18) |
23 (15) |
31 (20) |
30 (17) |
19 (11) |
|
R-ISS stage III, n (%) |
12 (9) |
16 (11) |
14 (10) |
16 (11) |
9 (6) |
|
t (4;14) or t(14;16) or del17 by FISH, n (%)* |
49 (35) |
46 (34) |
38 (29) |
40 (28) |
44 (28) |
Efficacy
KRd_ASCT showed consistent PFS advantage compared with KCd_ASCT and KRd12 and across both standard and high-risk patients (Table 2).
The 3-year PFS following R1, with KRd_ASCT was nearly 85% in patients with International Staging System (ISS) I, standard-risk fluorescence in situ hybridization (FISH), and lactate dehydrogenase (LDH) ≤ upper limit of normal (ULN). In patients with ISS II/III, high-risk FISH, and LDH > ULN, the 3-year PFS was nearly 70% with KRd_ASCT.
Premaintenance response rate following R2 was consistent across both arms; ≥ complete response (CR) was 62% vs 59%, and stringent CR was 50% vs 48% in KR and R, respectively.
At R2, 3-year PFS was significantly longer with KR maintenance compared with R alone (p = 0.026). The benefit of KR was observed in most subgroups.
The 3-year OS was around 90% in both arms, without any significant differences at this point.
Table 2. Progression-free survival1
|
PFS |
KRd_ASCT vs KCd_ASCT |
KRd_ASCT vs KRd12 |
KR vs R |
|---|---|---|---|
|
ASCT, autologous stem cell transplant; C, cyclophosphamide; d, dexamethasone; FISH, fluorescence in situ hybridization; HR, hazards ratio; ISS, International Staging System; K, carfilzomib; LDH, lactate dehydrogenase; PFS, progression-free survival; R, lenalidomide; R-ISS, Revised International Staging System; ULN, upper limit of normal. |
|||
|
3-year PFS rates, % |
78 vs 58 |
78 vs 66 |
75 vs 66 |
|
Overall HR (95% CI); p value |
0.53 (0.37–0.77); < 0.001 |
0.64 (0.44–0.94); 0.023 |
0.63 (0.42–0.95); 0.026 |
|
ISS |
|||
|
I, HR (95% CI); p value |
0.47 (0.26–0.85); 0.62 |
0.56 (0.31–1.03); 0.58 |
0.55 (0.30–1.00); 0.528 |
|
II/III, HR (95% CI) |
0.57 (0.35–0.93) |
0.70 (0.43–1.15) |
0.71 (0.40–1.26) |
|
FISH |
|||
|
Standard, HR (95% CI); p value |
0.52 (0.30–0.91); 0.81 |
0.57 (0.32–1.01); 0.80 |
0.61 (0.34–1.10); 0.946 |
|
High, HR (95% CI) |
0.47 (0.26–0.84) |
0.51 (0.28–0.94) |
0.59 (0.30–1.18) |
|
LDH |
|
|
|
|
≤ ULN, HR (95% CI); p value |
0.56 (0.36–0.86); 0.34 |
0.66 (0.43–1.01); 0.42 |
0.64 (0.40–1.02); 0.989 |
|
> ULN, HR (95% CI) |
0.36 (0.16–0.81) |
0.44 (0.18–1.05) |
0.65 (0.23–1.82) |
Safety
Safety at the R2 maintenance period was good, with similar number of patients requiring treatment discontinuation due to toxicity or R dose reduction. Grade III–IV hematologic adverse events (AEs) and serious adverse events (SAEs) were similar in KR arm compared with R alone (Table 3).
Table 3. Adverse events at R21
|
AEs at R2 |
KR |
R |
|---|---|---|
|
AEs, adverse events; DVT, deep vein thrombosis; K, carfilzomib; NA, not available; PE, pulmonary effusion; R, lenalidomide; R2, Randomization 2; SAEs, serious adverse events. |
||
|
Treatment discontinuation, % |
10 |
9 |
|
Lenalidomide dose reduction, % |
23 |
29 |
|
Carfilzomib dose reductions, % |
20 |
NA |
|
Grade III–IV hematologic AEs and SAEs |
||
|
Thrombocytopenia, % |
3 |
3 |
|
Neutropenia, % |
18 |
21 |
|
Anemia, % |
2 |
1 |
|
At least one hematologic AE, % |
22 |
23 |
|
Grade III–IV extra hematologic AEs and SAEs |
||
|
Dermatologic, % |
2 |
1 |
|
Thrombotic microangiopathy, % |
3 |
0 |
|
Gastrointestinal, % |
5 |
2 |
|
Infections, % |
4 |
7 |
|
Hepatic, % |
1 |
1 |
|
DVT/PE, % |
1 |
1 |
|
Hypertension, % |
3 |
0 |
|
Cardiac, % |
4 |
1 |
|
At least one extra hematologic AE |
27 |
15 |
MRD assessment (Aim 2)
Stefania Olivia et al.2 evaluated the rate of conversion from minimal residual disease-positivity (MRD-pos) to MRD-negativity (MRD-neg) during the maintenance phase, as well as PFS and overall survival (OS) of MRD-neg patients in different subgroups including all treatment arms.
Method
Centralized MRD assessment was performed in patients achieving ≥ very good partial response (VGPR) and every 6 months during maintenance until progression with both:
- 8-color, second-generation multiparametric flow cytometry (MFC), with a sensitivity of 10−5
- next-generation sequencing (NGS) of clonal immunoglobulin gene segments, with a sensitivity of 10−5
Efficacy
At median follow-up of 45 months after R1, 80% of patients were MRD-neg by MFC and 83% by NGS, indicating a lower risk of progression or death for MRD-neg patients across subgroups. After R2, 46% of MRD-positive patients turned negative in KR vs 32% in R (p = 0.04).
The OS at 3 years for MRD-neg patients was significantly higher than MRD-pos patients assessed with both methods: 96% vs 79% (MFC), and 97% vs 82% (NGS), p < 0.001.
Despite similar overall response rate and MRD-neg rates premaintenance, the rate of 1-year sustained MRD with MFC at the premaintenance endpoint was significantly higher in KRd_ASCT (68%) compared with KRd12 (54%), and KCd_ASCT (45%). Patients with 1-year sustained MRD-neg, using either MFC or NGS (10−5), represent a very favorable subgroup, with a 4-year PFS rate of 88% and 94%, respectively.
PFS analysis for MRD-pos and MRD-neg patients before maintenance showed that KRd_ASCT achieved higher 3-year PFS rates when compared with KCd_ASCT and KRd12, and the difference was particularly favorable for MRD-neg patients (Table 4).
A significantly extended PFS was observed in premaintenance MRD-neg patients who were randomized to KR vs R, assessed by both MFC and NGS; MRD-pos patients showed similar results (Table 4). Higher rates of 1-year sustained MRD-neg were also observed with KR.
Table 4. PFS in ITT patients according to premaintenance MRD stratified by treatment arm2
|
3-year PFS |
MFC |
NGS |
||
|---|---|---|---|---|
|
MRD-neg |
MRD-pos |
MRD-neg |
MRD-pos |
|
|
ASCT, autologous stem cell transplant; C, cyclophosphamide; d, dexamethasone; HR, hazards ratio; ITT, intention to treat; K, carfilzomib; MFC, multiparametric flow cytometry; MRD, minimal residual disease; neg, negative; NGS, next-generation sequencing; PFS, progression-free survival; pos, positive; R, lenalidomide. |
||||
|
KRd_ASCT vs KRd12, % |
88 vs 76 (HR 0.48; p = 0.01) |
62 vs 51 (HR 0.83; p = 0.46) |
91 vs 75 (HR 0.42; p = 0.08) |
68 vs 51 (HR 0.66; p = 0.07) |
|
KRd_ASCT vs KCd_ASCT, % |
88 vs 75 (HR 0.5; p = 0.04) |
62 vs 45 (HR 0.72; p = 0.15) |
91 vs 83 (HR 0.49; p = 0.19) |
68 vs 58 (HR 0.59; p = 0.01) |
|
KR vs R, % |
87 vs 71 (HR 0.51; p = 0.02) |
72 vs 61 (HR 0.73; p = 0.31) |
89 vs 71 (HR 0.38; p = 0.03) |
75 vs 64 (HR 0.64; p = 0.12) |
Conclusion
PFS significantly improved in patients treated with KRd_ASCT, compared with KRd12 and KCd_ASCT. KR maintenance also improved PFS and induced a high conversion rate from MRD-pos to MRD-neg using both techniques, MFC and NGS.
Similar PFS results were seen in patients with sustained 1-year MRD-neg using MFC and NGS, independent from treatment. The findings show consistency between the two techniques, MFC and NGS, indicating a high degree of agreement.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

