All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Patient treatment preferences for the management of MM
Measures of success in the management of multiple myeloma (MM) are typically considered in terms of disease status and clinical outcomes, such as survival and response. However, many MM therapies result in adverse events and side effects that can impact health-related quality of life (HRQoL).
Tervonen et al. published a report from an investigation of patient preferences on treatments in MM, based on their current health state. Below, we summarize the key findings.
Study design1
This study included a preference survey and discrete choice experiment. A total of 300 patients were included, comprising newly diagnosed transplant eligible (n = 108), ineligible (n = 105) and relapsed/refractory (n = 87) patients. The initial survey assessed preferences for eight distinct attributes, which are highlighted in Figure 1, alongside willingness to tolerate negative effects for an improvement in either disease or health state.
Figure 1. Attributes which influenced patient preferences*
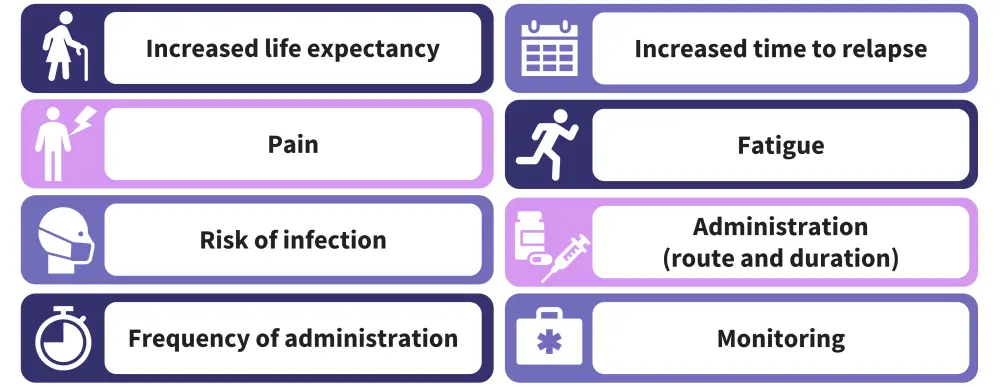
*Data from Tervonen, et al.1
Based on these attributes, a discrete choice task was delivered requiring patients to select their choice of two therapeutics, based on their influence on said factors. An example of a choice task is provided in Figure 2.
Figure 2. Example of a choice task to establish patient preference*
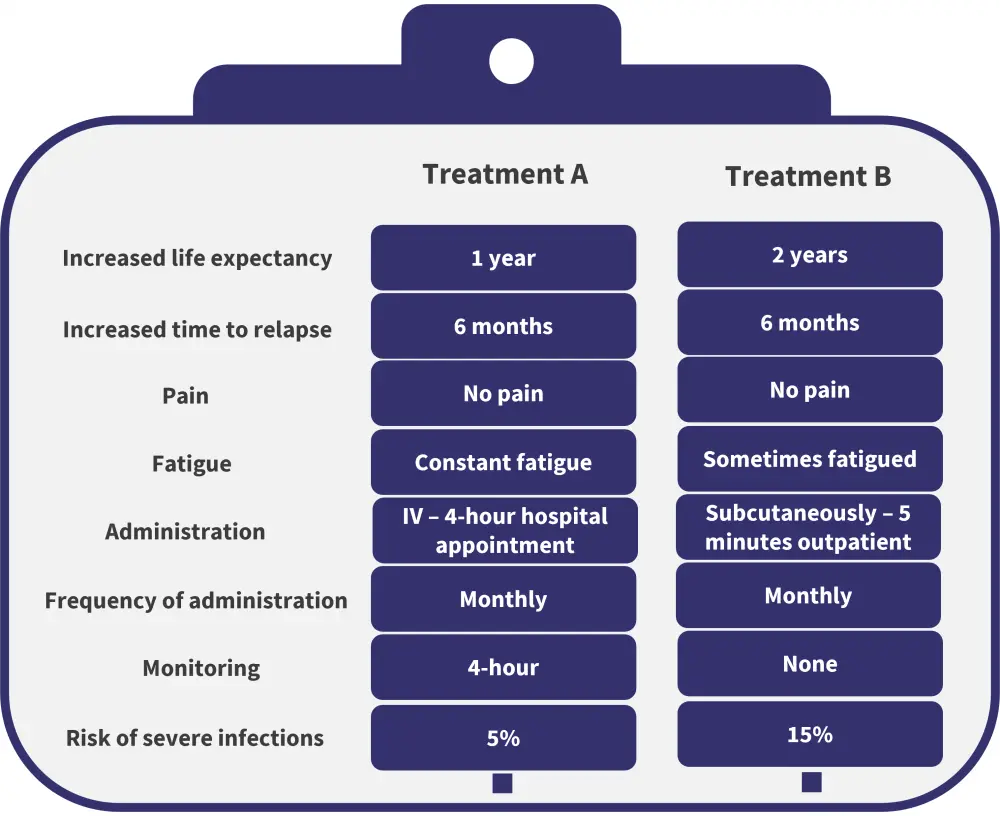
IV, intravenous.
*Data from Tervonen, et al.1
Results1
Data from the surveys were analyzed using a maxlimum likelihood estimate, this utilized patient-reported data to determine the parameters of the data distribution. The overall most valued attribute was a lack of pain, valued at a maxlimum likelihood estimate of 1.936 compared with baseline 0 for extreme pain. This was followed by increased life expectancy and lack of fatigue. The route and duration of treatment administration was the least valued of the attributes identified. Figure 3 outlines the value placed on an improvement in attributes compared with a given standard.
Figure 3. Attributes most valued by patients*
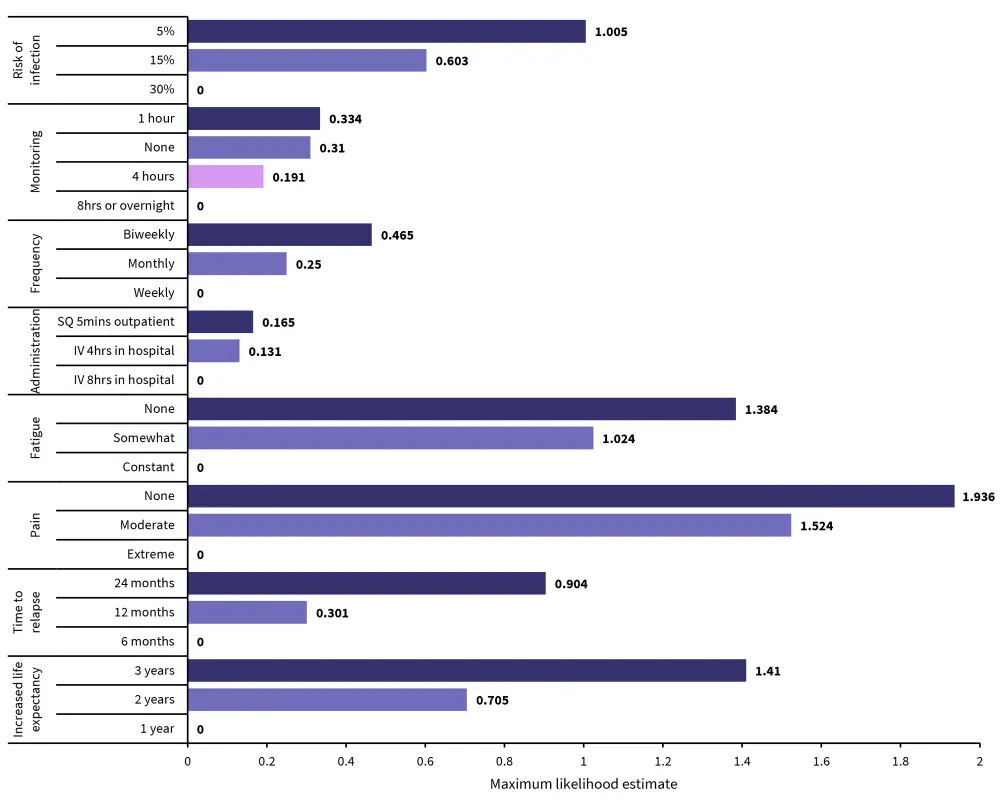
IV, intravenous; SQ subcutaneous.
*Data from Tervonen, et al.1
To tolerate extreme pain, patients wanted a minimum 2.8-year increase in their life expectancy and at least a 2-year increase to tolerate constant fatigue. Patients prioritized an increase in time to relapse over an increase in life expectancy, expressing willingness to tolerate a 0.9-year decrease in life expectancy for an extra 1 year before relapse. The minimum acceptable increase in life expectancy to tolerate disease characteristics is outlined in Figure 4, with baseline for comparison being no pain, no fatigue, IV 8 hours in hospital, weekly, and 8 hours monitoring. In addition to this, patients expressed that their willingness to tolerate pain depended on their current health state, with those in overall better health willing to tolerate more pain for an overall smaller increase in life expectancy.
Figure 4. Minimum acceptable increase in life expectancy by attribute*
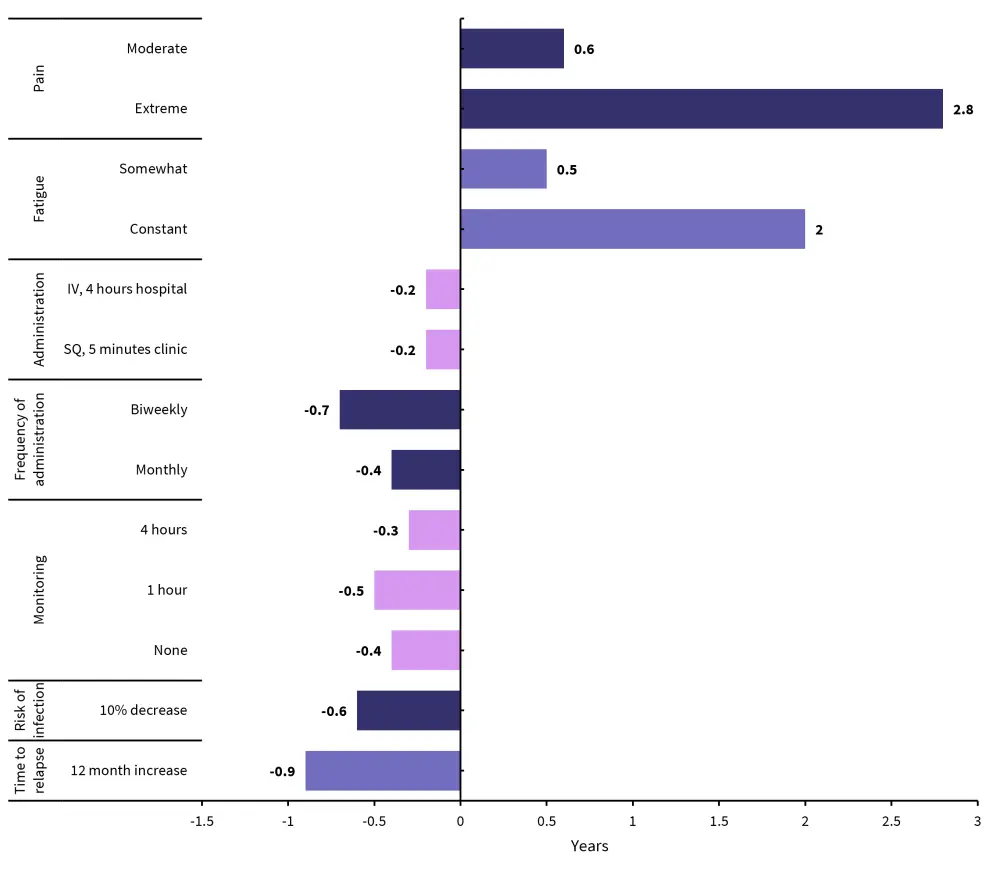
IV, invtravenously; SQ subcutaneously;
*Data from Tervonen, et al.1
Conclusion
Overall, patients prioritized pain above all other health outcomes, requiring a significant increase in life expectancy to tolerate extreme pain. Willingness to tolerate a negative impact on HRQoL correlated with current health state, indicating that current health state should factor into the choice of treatment. Data from this report emphasizes the importance of shared decision-making in MM treatment, particularly surrounding treatments that have an impact on health status and quality of life. The personal preferences of the patient are vital for a holistic approach to treating both the disease and the individual.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



