All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Novel bispecific antibodies in MM: characteristics and latest data
Bispecific antibodies (bsAbs) are a form of T-cell engaging immunotherapy that targets specific surface antigens on myeloma cells as well as the patient’s own T cells, causing direct T-cell activation and tumor cell death. The efficacy rates associated with these agents are markedly high; however, they are also associated with significant adverse events and toxicity.1
Here, we summarize a presentation by Einsele1 delivered at the International Myeloma Society (IMS) 5th Immune Effector Cell Workshop 2024 on novel bsAbs in multiple myeloma (MM) and how the unique characteristics (Figure 1) of these drugs may help overcome the challenges associated with bsAb treatment.
Figure 1. Structures of linvoseltamab, ABBV-383, alnuctamab, and cevostamab*
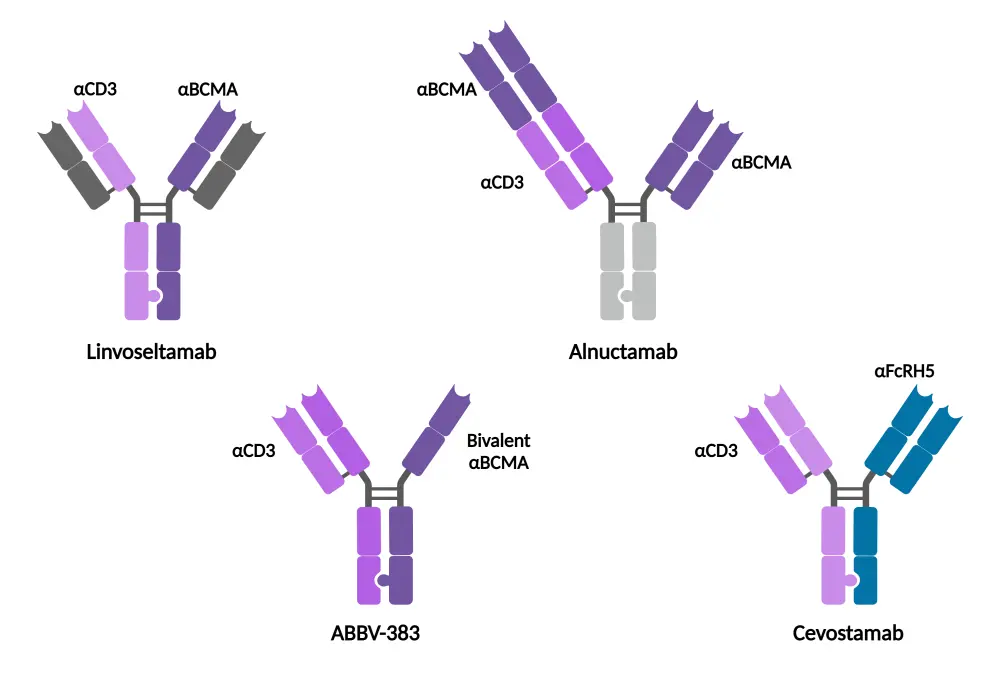
BCMA, B-cell maturation antigen; FcRH5, Fc receptor-homolog 5.
*Adapted from Einsele.1 Created with BioRender.com.
Linvoseltamab1
- Linvoseltamab is an investigational bsAb that targets B-cell maturation antigen (BCMA) and CD3 on the patient’s T cells.
- This drug also possesses an anti-albumin domain on the fragment crystallizable (Fc) region, which allows for a reduced dosing frequency.
- Dosing was reduced to once every 4 weeks (Q4W) as part of the phase I/II LINKER-MM1 (NCT03761108) clinical trial.
- Topline data from the Q4W dosing schedule in LINKER-MM1 include:
- An overall response rate (ORR) of 69.2%.
- Median progression-free survival (PFS) not reached.
- Any-grade infection rate of 69.2% and 36.2% at Grade 3/4.
ABBV-3831
- ABBV-383 is a BCMA-directed bsAb, uniquely possessing two BCMA binding domains and a silenced-FC backbone, designed to extend the half-life of the drug for reduced dosing.
- Dosing frequencies of once every 3 weeks (Q3W) or Q4W were investigated for their impact on CRS rates, with a 60 mg Q4W schedule resulting in the lowest overall CRS rate at 43%.
- No incidence of Grade 3 CRS was reported in this cohort.
- The lowest rates of Grade 3/4 infections were recorded in the Q4W cohort, at 10% compared with 34% in the 60 mg Q3W cohort.
- Q4W saw the highest ORR at 65% vs 60% in Q3W, as well as a median PFS not reached vs 13.7 months, respectively.
Alnuctamab
- Alnuctamab possesses bivalent binding to BCMA with a low CD3-binding domain and modified Fc region, mediating both reduced dosing and lower rates of cytokine release.
- A Q4W dosing schedule at the 30 mg target dose yielded:
- An ORR of 67%.
- A median PFS of 11.4 months, compared with 10.1 months in all target doses.
- A 12-month PFS rate of 45%, vs 44% in all target doses.
Cevostamab
- Cevostamab targets Fc receptor-homolog 5 (FcRH5), which is expressed on myeloma cells with near 100% prevalence. FcRH5 is also highly expressed at all stages of B-cell maturation and is located near the chromosomal breakpoint.
- Clinically, cevostamab was evaluated in the heavily pre-treated population, with a median of six prior lines of therapy.
-
- The ORR in the 132–198 mg dose level was 56.7% vs 36.1% in the 20–90 mg dose level.
- None of the patients who experienced a stringent complete response had relapsed by data cut-off.
|
Key learnings |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




