All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Infections following bispecific antibody therapy in MM: incidence and characteristics
Bispecific antibodies are becoming increasingly central in the treatment of many patients with relapsed/refractory multiple myeloma (RRMM). However, an increased risk of severe infection, including those resulting in death, has been reported from emerging clinical trials of bispecific antibodies. An understanding of the nature of infection, including localization, pathogens, and risk factors, is vital to better manage these infections, improve survival, and improve patient quality of life.
Here, we summarize a multicenter, retrospective study, conducted in France by Jourdes et al. and published in the journal Clinical Microbiology and Infection, on the incidence and characteristics of infections following treatment with bispecific antibody therapy.
Methods
- Patients treated with bispecific antibody therapies were retrospectively assessed for subsequent infections.
- Data were collected on patient characteristics, localization, prophylaxis, and prior treatment, and analyzed for association with infection incidence and severity.
Results
Incidence of infection
- A total of 229 patients treated with bispecific antibodies were included (Figure 1).
- 234 infections were recorded, with 123 (53%) of these at Grade 3 or higher.
- The hospitalization rate was 56%, with 13% resulting in admission to the intensive care unit and 9% in death.
- Across all patients, the incidence of first infection was 70%:
- 73% in patients treated with B-cell maturation antigen (BCMA)-directed therapies; and
- 51% in patients treated with G-protein-coupled receptor class C group 5 member D (GPRC5D)-directed therapies.
- All infections of Grade 4 or 5, as well as those requiring admission to intensive care, were identified in patients treated with BCMA-directed bispecific antibodies.
Figure 1. Patient population and prior bispecific antibody therapy*
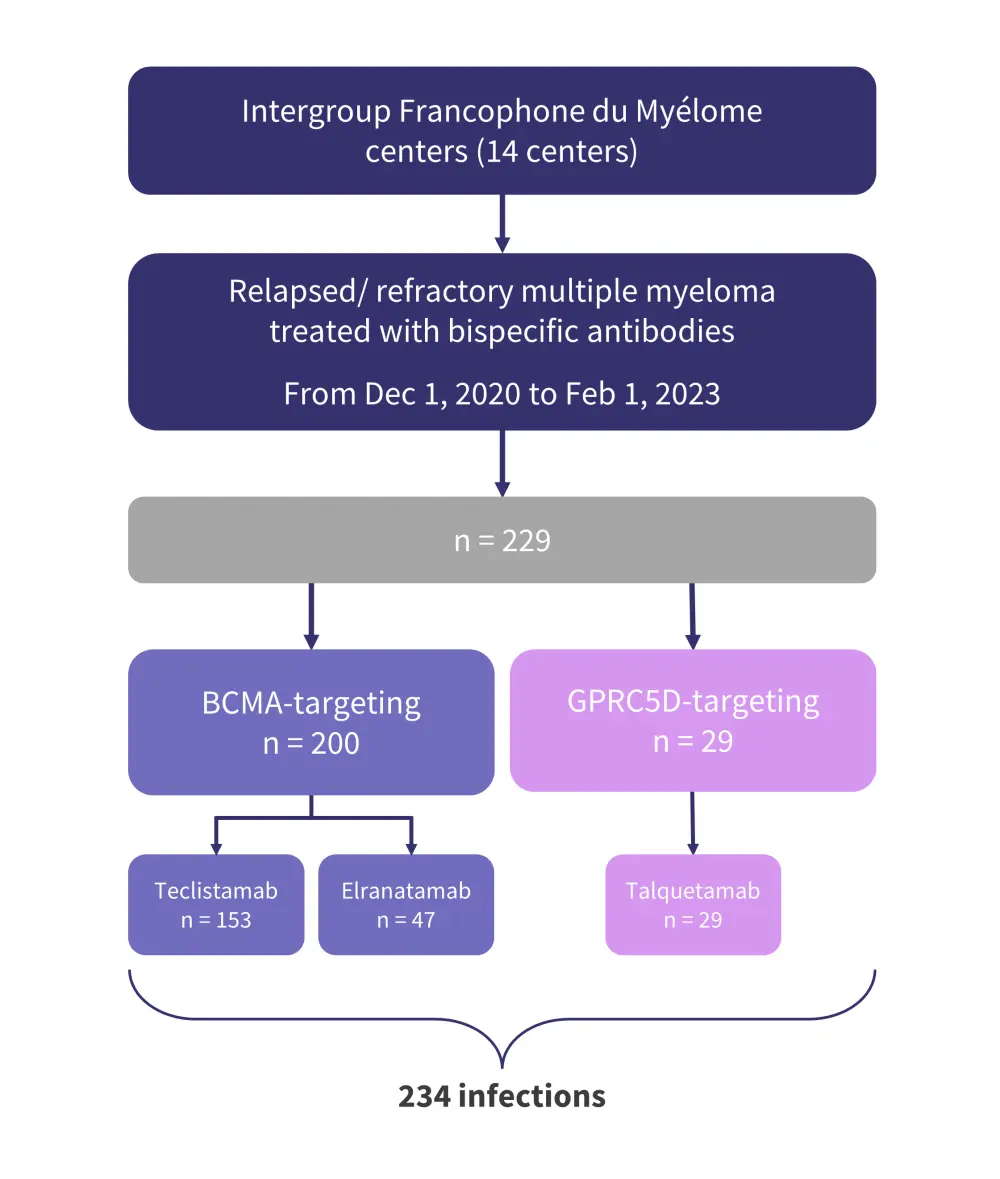
BCMA, B-cell maturation antigen; GPRC5D, G-protein-coupled receptor class C group 5 member D.
*Adapted from Jourdes, et al.1
Characteristics of infection
- Several infection sites were observed, with the most common being the lower respiratory tract, accounting for 41% of all infections (Figure 2).
- This was followed by systemic infections (22%), which are particularly challenging to treat if not identified early.
Figure 2. Site of infection following treatment with bispecific antibody therapy*
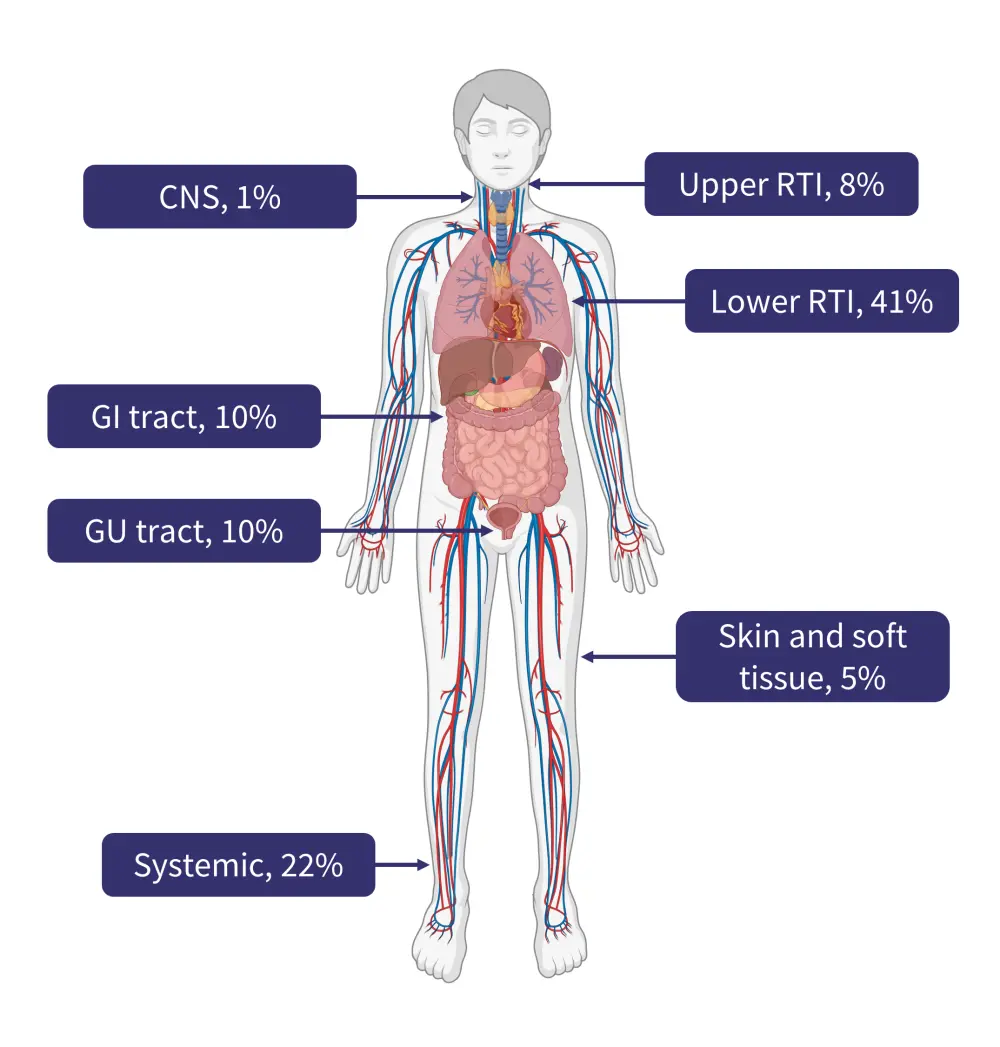
CNS, central nervous system; GI, gastrointestinal; RTI, respiratory tract infection.
Created with BioRender.com.
*Data from Jourdes, et al.1
- The most common infections were bacterial, accounting for 56% of all infections, followed by viral, and a small number of fungal and parasitic infections (Figure 3A).
- Of the bacterial, viral, and fungal infections, a single pathogen was responsible for over half of its respective category (Figure 3B).
- The most common pathogens were:
- Bacterial; Enterobacteriaceae 52%
- Viral; respiratory virus 63%
- Fungal; Aspergillus spp. 75%.
Figure 3A. Pathogen distribution of infections following treatment with bispecific antibody therapy*
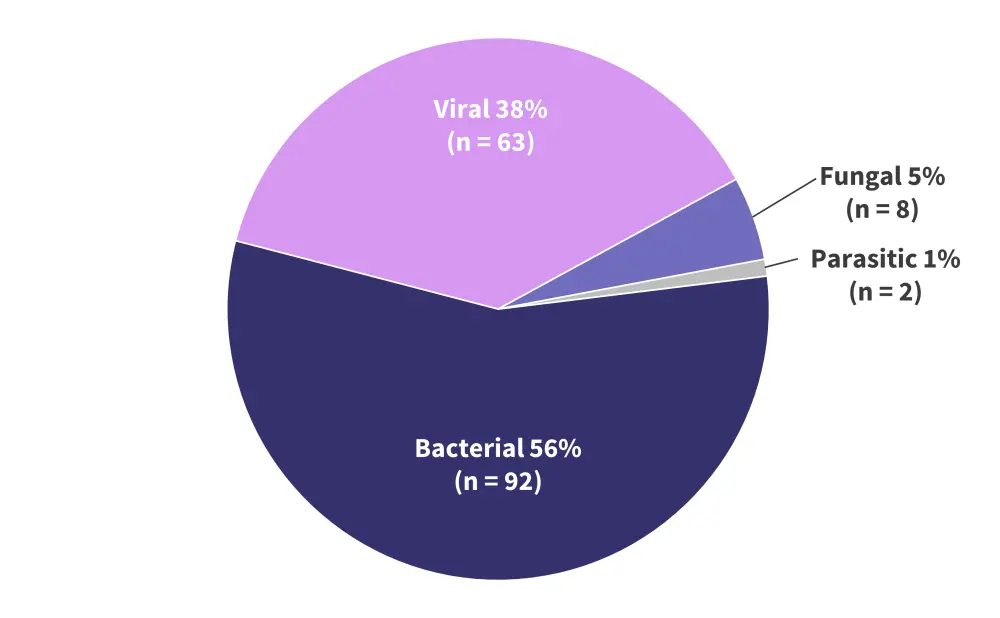
*Data from Jourdes, et al.1
Figure 3B. Pathogens identified underlying infections following treatment with bispecific antibody therapy*
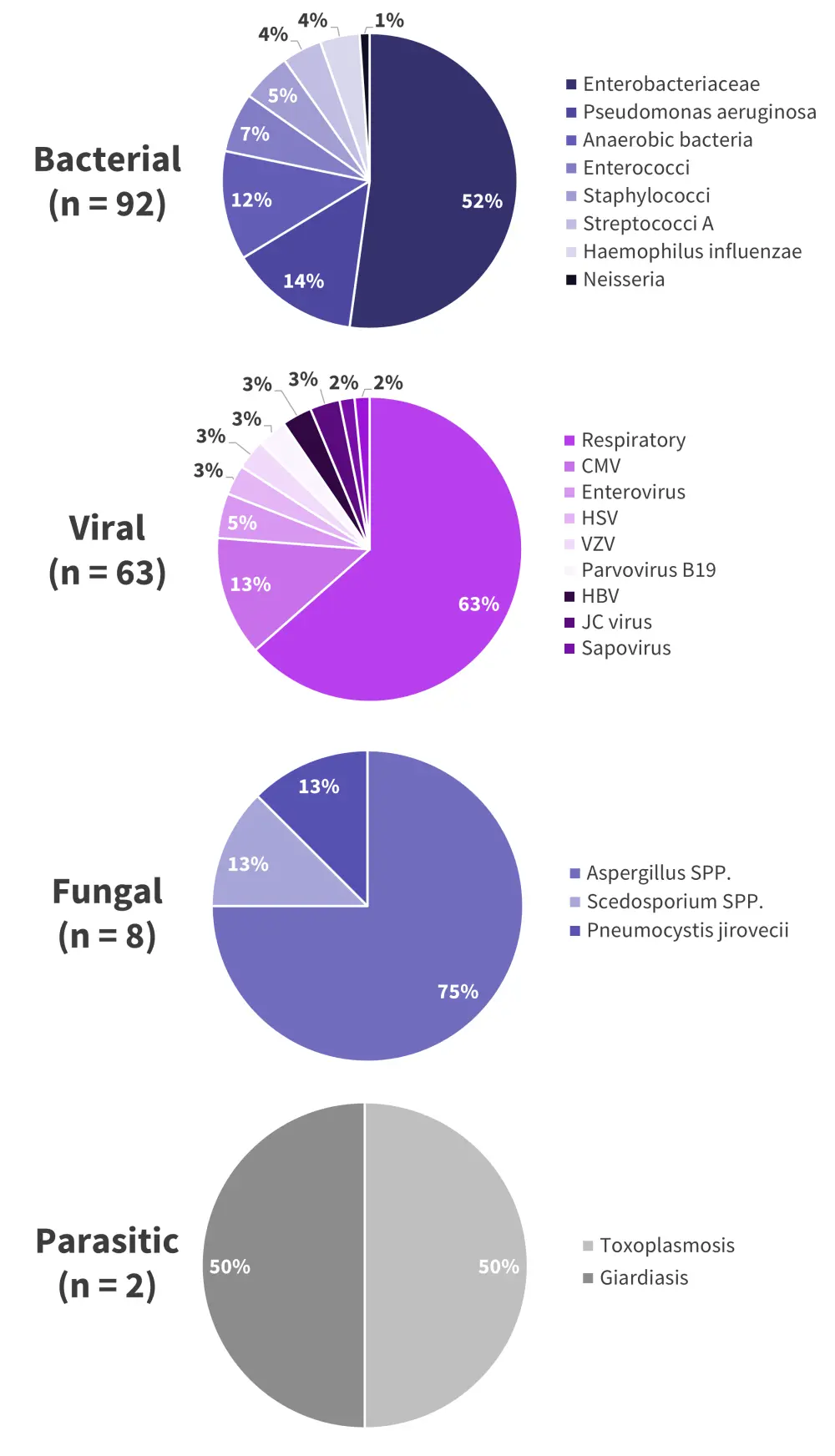
CMV, cytomegalovirus; HBV, hepatitis B virus; HSV, herpes simplex virus; JC, John Cunningham; SPP, several species; VZV, varicella zoster virus.
*Data from Jourdes, et al.1
Risk factors and impact on treatment
- The use of corticosteroids for the treatment of cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) was the only factor associated with an increased risk of first infection, with a hazard ratio (HR) of 2.13 (95% confidence interval [CI]: 1.38–3.28)
- GPRC5D-targeted bispecific antibodies resulted in the lowest overall incidence of infection at HR 0.53 (95% CI: 0.3–0.94)
- Antibacterial prophylaxis also reduced the likelihood of infection at HR 0.65 (95% CI: 0.46–0.9)
- The majority of infections (61%) occurred in patients with hypogammaglobulinemia (<400 mg/dL) and in 21% of those undergoing immunoglobulin substitution.
- Infection had an impact on the course of treatment in 44% of patients, resulting in treatment discontinuation in 13% and a delay of 14 or more days in administration in 30%.
Conclusion
Overall, the majority of patients treated with bispecific antibodies developed an infection, with 53% of infections at Grade 3 or higher, 44% resulting in treatment modification, and 9% in death.
The influence of severe infections on patient outcomes and quality of life is considerable, highlighting this group as key for research into prevention strategies. Patients also treated with corticosteroids are a particularly high-risk group, warranting careful observation and rational consideration of treatment strategies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



