All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
EMN recommendations: Prevention and management of CAR-T and bispecific antibody-related adverse events in MM
Do you know... Which of the following is/are common severe adverse event(s) associated with patients with multiple myeloma who are treated with chimeric antigen receptor (CAR) T-cell therapy or T cell-redirecting bispecific antibodies (bsAbs)?
Patients with relapsed/refractory multiple myeloma (MM) may be treated with chimeric antigen receptor (CAR) T-cell therapy or T cell-redirecting bispecific antibodies (bsAbs).1 Results from clinical trials have shown these therapies to be efficacious, and they are under investigation as first-line treatment options for patients with MM.1 With this advancing therapeutic landscape, the European Myeloma Network (EMN) has provided recommendations for the prevention and management of adverse events (AEs) commonly associated with these treatments.2 The Multiple Myeloma Hub is pleased to summarize this consensus here.
General recommendations
The most common severe AEs associated with patients treated with bsAbs or CAR T-cell therapy are cytokine release syndrome (CRS), neurotoxicity, cytopenia, infections, and hypogammaglobulinemia.1 At treatment initiation, patients should be screened for active infections, organ and bone marrow function, and comorbidities. At the step-up dosing stage of either treatment option, patients should be monitored for signs and symptoms of toxicity and the dose should be adjusted according to individual patient response.1
CRS
In patients treated with bsAbs or CAR T-cell therapy, CRS is generally observed after initial exposure, but can also occur post step-up doses.1 The signs and symptoms to look out for include fever, fatigue, headaches, myalgias, nausea, hypotension, hypoxia, and organ dysfunction. Managing these signs and symptoms varies according to the severity of the AE, but stepwise dosing should be considered (Figure 1). Macrophage activation syndrome is associated with concurrent CRS, and it is recommended that patients with fever, cytopenias, and hyperinflammatory markers should be monitored for both macrophage activation syndrome and Epstein-Barr virus.1
Figure 1. Recommendations for the management of CRS by grade of severity*
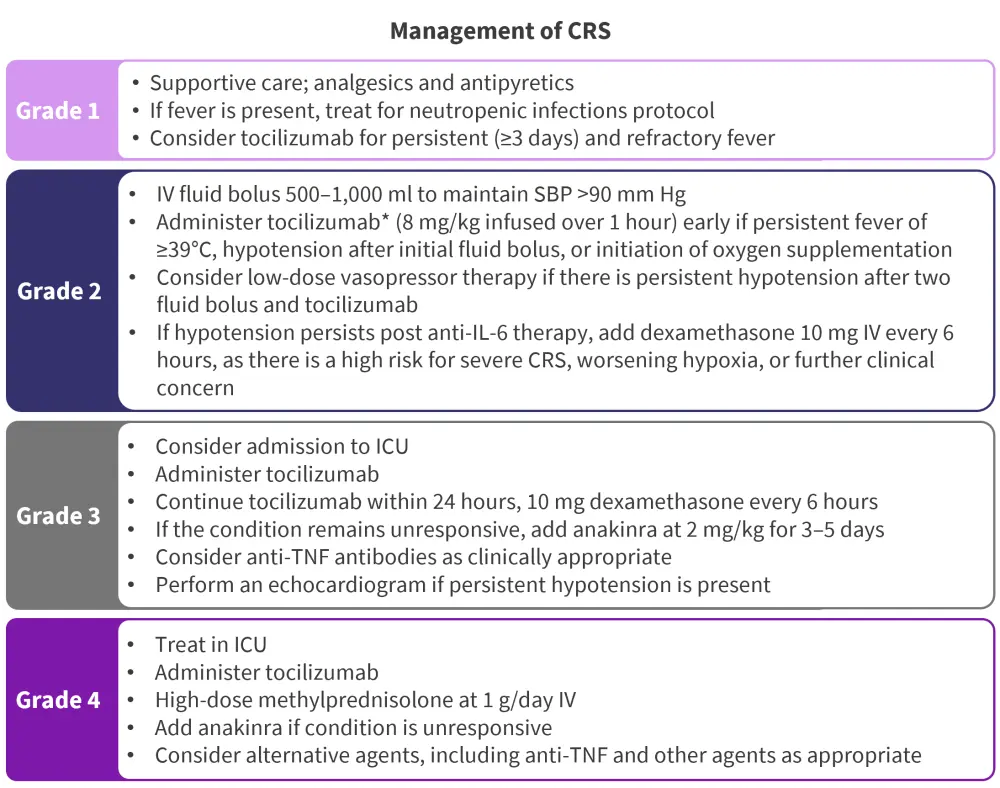
CRS, cytokine release syndrome; ICU, intensive care unit; IL, interleukin; IV, intravenous; SBP, systolic blood pressure; TNF, tumor necrosis factor.
*Adapted from Ludwig, et al.2
Neurotoxicity and ICANS
Neurotoxicity affects the central nervous system and can result in activating or deploying endogenous T cells.1 Immune effector cell-associated neurotoxicity syndrome (ICANS) is the second most common non-hematologic AE associated with CAR T-cell therapy, with an incidence of 2–64% for mild ICANS.2 Symptoms include word-finding difficulties, confusion, dysphasia, and aphasia. Although the biological mechanisms of neurotoxicity are not yet fully understood, pro-inflammatory cytokines produced by CAR T-cells are thought to be responsible for pathogenesis.2 ICANS is often associated with CRS; therefore, it is recommended that CRS prevention can help manage the development of ICANS.2 Patients who develop ICANS without concomitant CRS are advised to be switched to total parenteral nutrition and medication (Figure 2).2
Figure 2. Recommendations for the management of ICANS by grade of severity according to the American Society for Transplantation and Cellular Therapy*
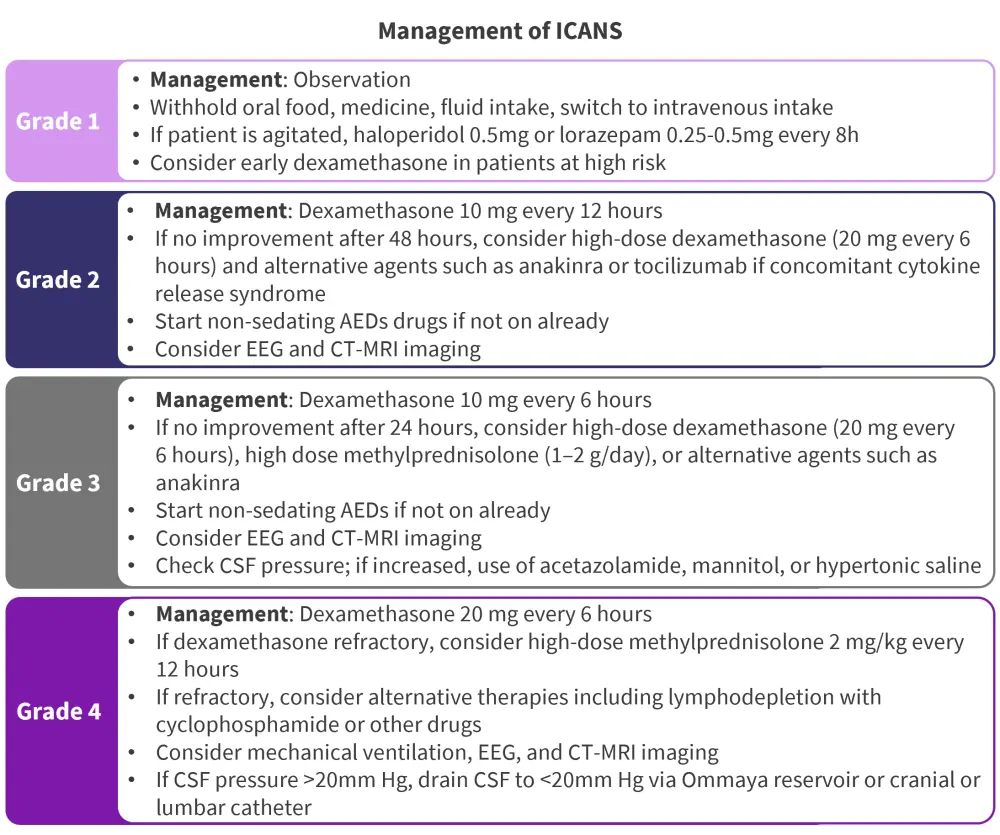
AED, antiepileptic drug; CRS, cytokine release syndrome; CSF, cerebrospinal fluid; CT-MRI, computed tomography-magnetic resonance imaging; EEG, electroencephalogram.
*Adapted from Ludwig, et al.2
Cytopenias and hypogammaglobulinemia
Cytopenias are common following CAR T-cell therapy and bsAb therapy, with incidence of Grade 3 or worse neutropenia recorded in 16–64% of patients receiving bsAbs and 55–95% of patients treated with CAR T-cell therapy.1 It is recommended that patients with Grade ≥3 neutropenia should receive granulocyte colony-stimulating factor.2 Dose administration is dependent on individual patient situation, and treatment initiation is not recommended for patients who have received bsAbs or CAR T-cell therapy within 14 days due to risk of inducing cytokine release.2
Hypogammaglobulinemia is a common AE in patients who have received B-cell maturation antigen therapy; a specific CAR T-cell therapy.1 Prophylactic administration of 400 mg/kg immunoglobulin (Ig)G at 1–4 weeks is recommended for patients with low IgG concentrations.2 Patients with higher IgG concentrations and recurrent bacterial infections who do not respond to antibiotic therapy can be considered for treatment with immunoglobulins, as this may result from an inability to mount an adequate antibody response.1
Prophylaxis and management of infections
Studies have shown infections are common in patients treated with bsAbs and CAR T-cell therapies; therefore, it is recommended that patients are screened at baseline for hepatitis B, hepatitis C, human immunodeficiency virus, cytomegalovirus, and Epstein-Barr infections.1 Cytomegalovirus, Epstein-Barr, and hepatitis B reactivation can occur after and during treatment; thus, testing should be considered on an individual case basis. Recommended preventative measures for prophylaxis and management of these infections include vaccination against varicella zoster virus with acyclovir or valaciclovir during treatment until 7 weeks post complete immune reconstitution (Figure 3).2
Figure 3. Recommendations for prophylaxis and management of infections*
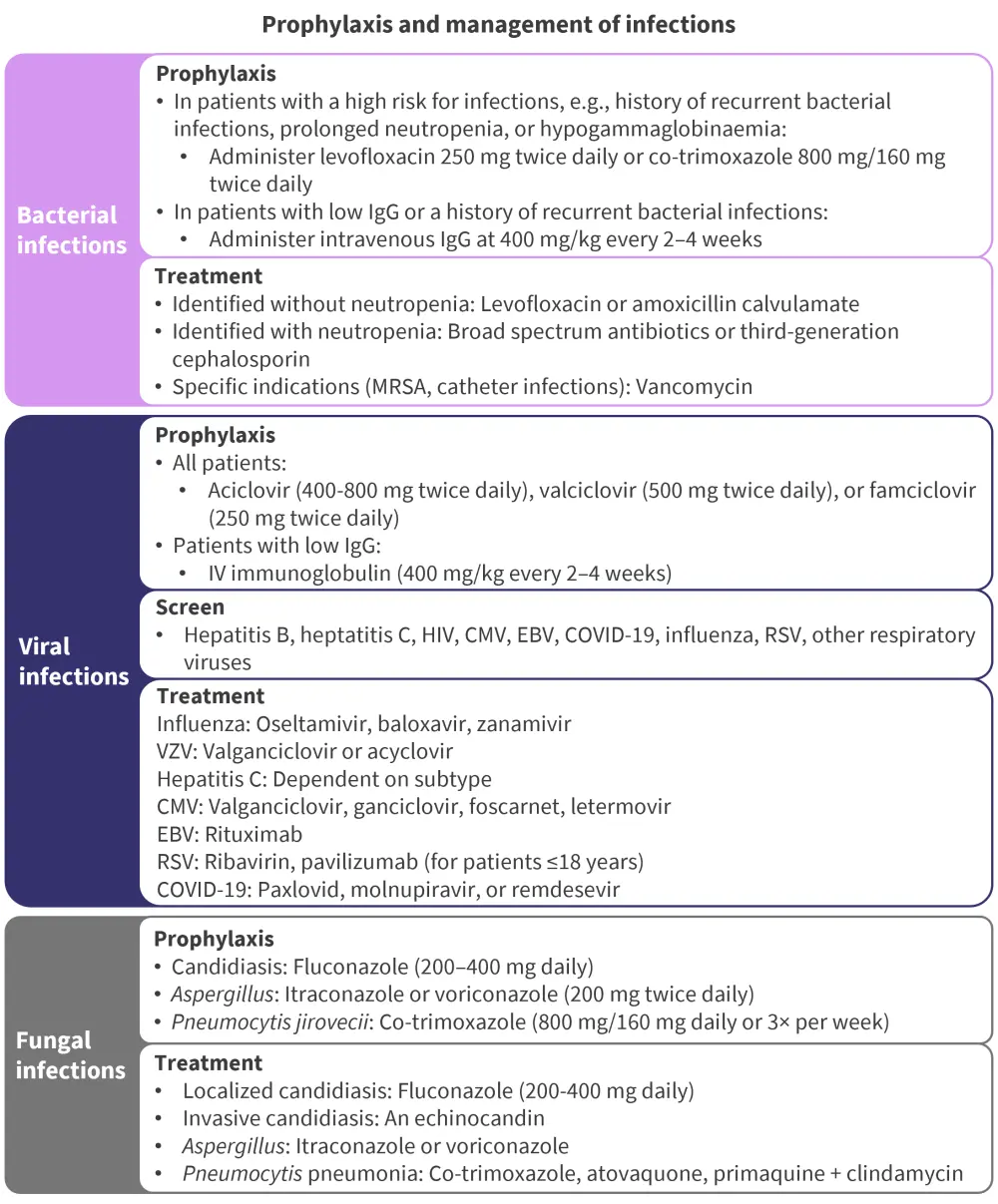
CMV, cytomegalovirus; EBV, Epstein-Barr virus; IgG, immunoglobulin G; MRSA, methicillin-resistant Staphylococcus aureus; RSV, respiratory syncytial virus; VZV, varicella zoster virus.
*Adapted from Ludwig, et al.2
Conclusion
The recommended measures summarized above, including premedication, frequent assessment for symptoms and severity of CRS, and step-up dosing, should be considered in patients with MM who are selected for first-line treatment with bsAbs or CAR T-cell therapy. This will ensure management and prevention of AEs associated with these therapies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

