All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
MRD in MM: Implications for clinical practice and trial design
Do you know... Which method of MRD assessment offers a minimally invasive option for long-term monitoring, after initial bone marrow-based assessment in the first year?
During the Multiple Myeloma Hub Steering Committee Meeting in April 2025, key opinion leaders met to discuss the implications of measurable residual disease (MRD) on clinical practice and trial design in multiple myeloma (MM). The meeting opened with a presentation by Bruno Paiva and featured a discussion including Paul Richardson, Hang Quach, Sonja Zweegman, and Rakesh Popat.
During their presentation, Paiva explored the evolving role of MRD in MM, highlighting both clinical and research applications. Paiva reviewed current MRD detection methods (Figure 1), evaluated sustained MRD negativity as an indicator for long-term survival outcomes (Figure 2), discussed the value of MRD as an early endpoint in clinical trials, and outlined scenarios where MRD could guide treatment decisions, such as fixed-duration therapy or reinitiation upon MRD resurgence.
MRD in MM: Implications for clinical practice and trial design
MRD in MM: Implications for clinical practice and trial design
Figure 1. Overview of detection methods for MRD negativity in MM*
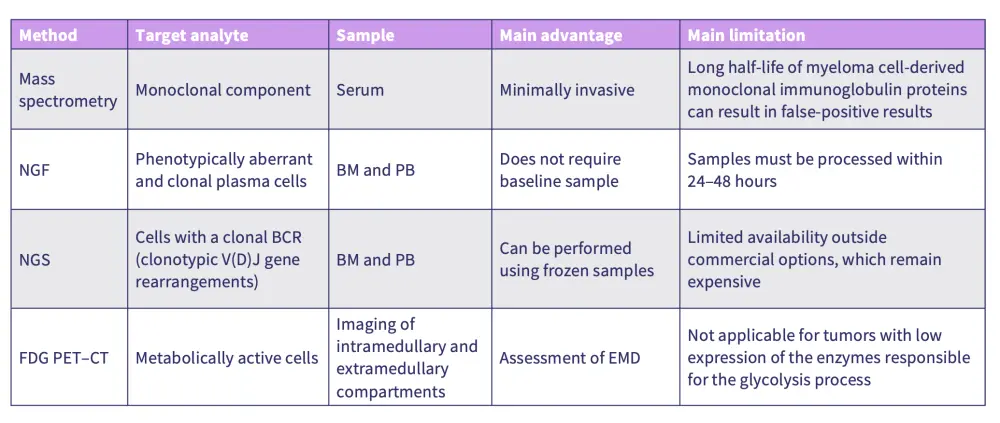
Figure 2. Relationship between efficacy, durable MRD, and survival*
Key learnings
There are multiple assessment methods for MRD, including mass spectrometry, next-generation flow, next-generation sequencing, and positron emission tomography-computed tomography (PET-CT).
Bone marrow remains the preferred sample type for MRD assessment in the first year of treatment due to the larger concentration of target cells leading to higher sensitivity.
Mass spectrometry offers a minimally invasive option for ongoing monitoring after initial bone marrow-based MRD assessments.
Sustained MRD negativity is associated with improved PFS and overall survival, making it a strong prognostic marker and a potential surrogate endpoint in clinical trials.
MRD assessment allows for early insights into treatment efficacy, enabling comparisons between regimens before long-term outcome data such as progression-free survival (PFS) are available.
Incorporating MRD as a clinical trial endpoint has the potential to significantly reduce required sample sizes and trial duration, therefore accelerating the evaluation and potential approval of novel therapies.
MRD status can also inform personalized treatment strategies, including guiding treatment-discontinuation in patients with sustained MRD negativity, reducing the risk of overtreatment.
Detecting MRD resurgence before clinical relapse could allow for earlier re-initiation of treatment.
The prognostic relevance of MRD may differ by patient subgroup, with more research needed to clarify its role in high-risk populations and in patients with atypical disease.
Advancing the clinical utility of MRD requires standardization of assessment, additional real-world data collection, and considered trial design to integrate MRD into the treatment of MM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Paul Richardson
Paul Richardson Sonja Zweegman
Sonja Zweegman Bruno Paiva
Bruno Paiva Hang Quach
Hang Quach Rakesh Popat
Rakesh Popat

