All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Monoclonal gammopathy of renal significance: Current classification, diagnosis, and management
The Multiple Myeloma Hub recently presented a visual abstract highlighting the key updates and changes in the 5th edition of the World Health Organization (WHO) classification of plasma cell neoplasms and paraprotein-producing disorders, as well as other hematolymphoid tumors.1,2
The latest WHO update reflects changes in the reclassification and reorganization of some existing conditions and includes a new section on monoclonal gammopathies (MGs). The MG classification includes cold agglutinin disease, immunoglobulin M (IgM) and non-IgM monoclonal gammopathy of unknown significance (MGUS), and monoclonal gammopathy of renal significance (MGRS) as an entity in its own right.1,2 What do we know about this newly classified condition of MGRS?
MGUS versus MGRS: Differences and similarities
MGUS describes a benign plasma cell or B-cell proliferation disease that by definition causes no end-organ damage and requires no treatment (e.g., targeted agent or chemotherapy). The diagnosis and treatment of MGUS have previously been covered by Multiple Myeloma Hub. While benign, MGUS has premalignant potential; it can progress to other conditions such as Waldenstrom’s macroglobulinemia, chronic lymphocytic leukemia, or multiple myeloma.1,2 In the WHO 5th classification of IgM and non-IgM MGUS, there is no association of the condition with any end-organ damage and the classification stipulates that no bone lesions, renal insufficiency, anemia, or hypercalcemia can be attributed to the plasma cell clone underlying MGUS.1
Considering MGRS, the condition was first named in 2012 by the International Kidney and Monoclonal Gammopathy (IKMG) Research Group, recognized as a hematologic disorder that produces a monoclonal paraprotein associated with renal damage.3 MGRS is by definition distinct to MGUS, in that it does cause end-organ damage, specifically in the form of kidney injury. The IKMG Research Group updated the definition of MGRS in 2017 to include any hematologic condition with a nephrotoxic monoclonal paraprotein that causes renal damage.4 However, as with MGUS, the plasma clone causing MGRS must not cause tumor-like complications or meet any malignant hematologic criteria that calls for therapeutic intervention.4
At the 18th International Myeloma Workshop (September 2021), Leung presented an overview of existing and new developments in the diagnosis and treatment of MGRS,5 which built on a review by Castillo et al.6 published earlier that year in the American Journal of Hematology.6 Here, we present a summary of the current classification, diagnosis, and management of MGRS.
Epidemiology, diagnosis, and classification of MGRS
The median age of MGRS diagnosis is ~60 years,2 with a higher incidence in males compared with females (75% vs 60%, respectively).2 Renal lesions occur at a higher incidence in patients with MGRS compared with patients with hematologic malignancies.7 Approximately 40% of all patients with a MG have MGRS as the underlying cause, with proteinuria ≥1.5 g/d, hematuria, and elevated serum free light chain ratio as the three most common laboratory indicators.8 Approximately 1.5% of patients diagnosed with MGUS have MGRS as their actual underlying disease, demonstrating the relative rarity of MGRS.9
According to an IMKG consensus report, a renal biopsy is required for the diagnosis of MGRS, with light microscopy and immunofluorescence microscopy evaluations considered fundamental to the classification of MGRS-associated renal lesions.6 Although deeper histological definitions of MGRS exist (Figure 1), MGRS can be broadly classified according to:
- the presence of immunoglobulin deposits;
- minor presence or absence of immunoglobulin and complement component 3 (C3) deposits; and
- the absence of deposits of either immunoglobulin or C3.6
In addition to this, the current WHO classification mandates that the following are essential for the diagnosis of MGRS:
- a kidney biopsy demonstrating monoclonal immunoglobulin mediated kidney injury;
- a progressive acute or subacute kidney injury; and
- a proteinuria defined as >1 g/day (predominantly albuminuria).2
Figure 1. Categorization of renal lesions in MGRS*
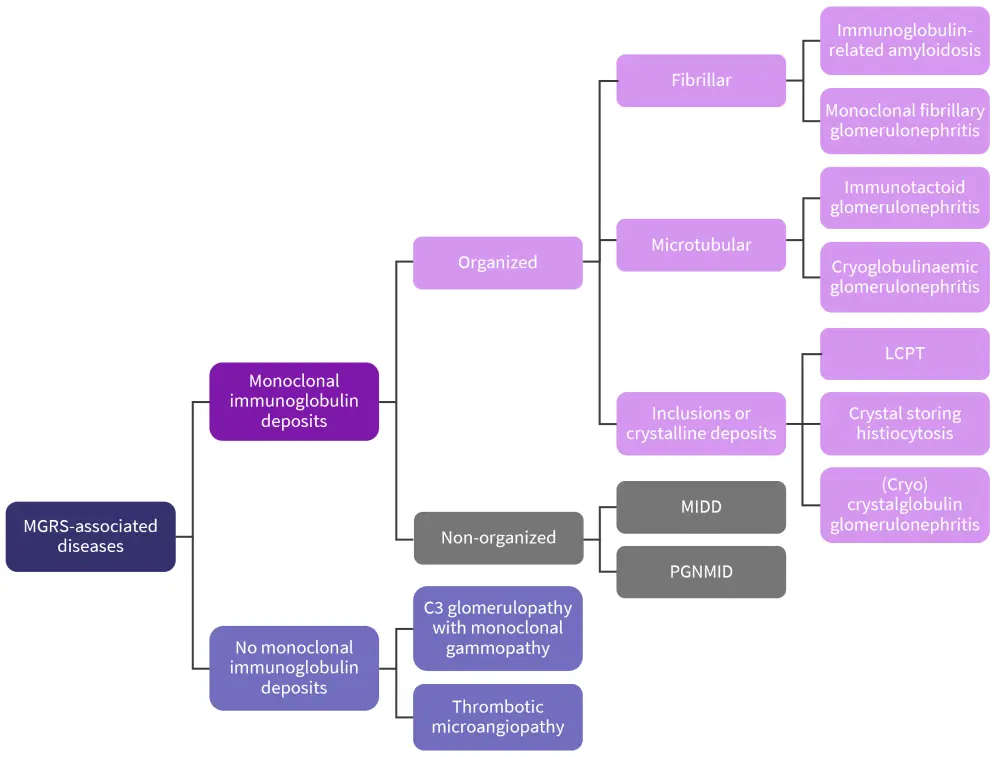
C3, complement component 3; LCPT, light-chain proximal tubulopathy; MGRS, monoclonal gammopathy of renal significance; MIDD, monoclonal immunoglobulin deposition disease; PGNMID, proliferative glomerulonephritis and monoclonal immunoglobulin deposits.
*Adapted from Leung et al.4
The diagnosis of MGRS should be confirmed by integrating the findings of the renal biopsy as well as patient’s medical history, bone marrow biopsy, imaging, and laboratory data.4,6 Further to the essential criteria for the diagnosis of MGRS, the recent WHO classification update included a revision of MGRS subtypes, according to immunoglobulin deposits, histological and immunological findings, and the presence of extrarenal manifestations (Table 1). These evaluations can help with the diagnosis and risk stratification of patients.
Table 1. MRGS subtypes according to the 5th edition of the WHO Classification of Haematolymphoid Tumours*
|
GBM, glomerular basement membrane; HC, heavy chain; Ig, immunoglobulin; LC, light chain; MG, monoclonal gammopathy; MIg, monoclonal immunoglobulin; MRGS, monoclonal gammopathy of renal significance; TMA, thrombotic microangiopathy. |
|||
|
Subtype |
Ig deposits |
Immunological and ultrastructural characteristics |
Extrarenal manifestations |
|---|---|---|---|
|
Always associated with MGRS |
|||
|
Crystal storing histiocytosis |
LC |
Intracellular LC crystals in interstitial histiocytes (with or without crystals in tubular and glomerular cells |
Yes |
|
Crystalglobulin-induced nephropathy |
Ig |
Extracellular MIg crystals within glomerular and vessel lumina (with or without TMA) |
Yes |
|
Ig-related amyloidosis |
LC, Ig, HC |
Extracellular deposition of Congophilic randomly-oriented fibrils |
Yes |
|
Light chain proximal tubulopathy |
LC |
Crystalline or non-crystalline LC inclusions within proximal tubular cells |
Yes |
|
Monoclonal immunoglobulin deposition disease |
LC, Ig, HC |
Finely granular “punctate” MIg deposits in tubular and glomerular basement membranes and mesangium |
Yes |
|
Proliferative glomerulonephritis with monoclonal immunoglobulin deposits |
Ig, LC |
Amorphous MIg deposits in mesangium and subendothelial zone (and occasionally in subepithelial zone) |
No |
|
Frequently associated with MGRS |
|||
|
C3 glomerulopathy with MG |
Only C3 |
C3 deposits in mesangium, subendothelial and subepithelial zones (and the lamina densa of the glomerular basement membranes in dense deposit disease) |
No |
|
Cryoglobulinemic glomerulonephritis (Type I and most II) |
Ig |
Short microtubular and annular deposits composed of MIg only or MIg and polyclonal Igs |
Yes |
|
Monoclonal immunotactoid glomerulonephritis |
Ig |
Microtubular deposits composed of MIg |
No |
|
Thrombotic microangiopathy with MG |
None |
Chronic endothelial cell injury, no MIg or C3 deposits |
Yes |
|
Rarely associated with MGRS |
|||
|
Monotypic membranous nephropathy |
Ig |
Subepithelial deposits of MIg |
No |
|
Monotypic anti-GBM disease |
Ig |
Linear deposits of MIg along the glomerular basement membranes, no electron dense deposits ultrastructurally |
No |
|
Monotypic IgA nephropathy/ Henoch Schönlein purpura nephritis |
Ig |
Monoclonal IgA deposits in the mesangium (and occasionally in subepithelial and/or subepithelial zones) |
No/yes |
In patients with immunoglobulin deposits, immunoglobulin light chain restriction is diagnostic of MGRS.4 Heavy chain restriction is supportive of MGRS diagnosis but is not a diagnostic tool on its own, unless used for diseases that present involvement of immunoglobulin heavy chain only (e.g., heavy-chain deposition disease or immunoglobulin heavy-chain amyloidosis).4 Of note, C3 dominant deposits are found in patients with a MG and no deposits are found in patients with thrombotic microangiopathy with a MG.4
Treatment of MGRS lesions
For decisions on treatment, it is anticipated that the underlying clone has been identified as part of the diagnosis of MGRS.10 For certain MGRS subtypes, the main approach to the treatment is to watch and wait, monitoring for signs of disease progression or increased renal dysfunction.10 Leung et al. propose an algorithm that can be used to guide management and treatment decisions for patients with MGRS, up to and including the point of MGRS malignant progression (Figure 2).3
Figure 2. Treatment algorithm for MGRS lesions*
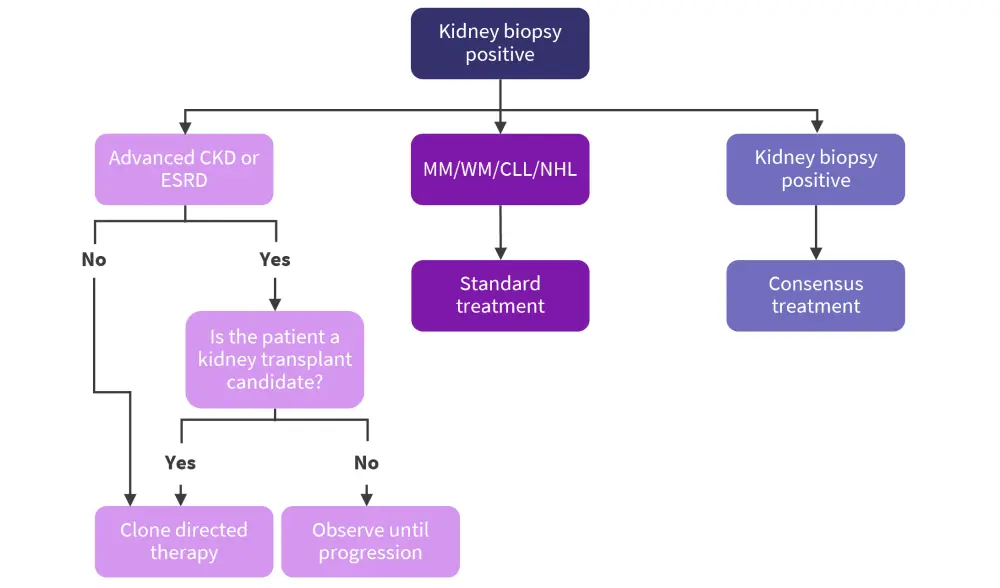
AL, amyloid light-chain; CKD, chronic kidney disease; CLL, chronic lymphocytic leukemia; ESRD, end stage renal disease; MGRS, monoclonal gammopathy of renal significance; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; WM, Waldenstrom’s macroglobulinemia.
*Adapted from Leung.5
It has been recognized that the monoclonal immunoglobins deposited through the disease pathophysiological process do not respond to immunosuppressive treatment, unlike other nephropathies.11 MGRS relapse can occur with subsequent and progressive damage to the transplanted kidney(s) even after renal transplant secondary to end-stage renal failure.11 Subsequently, while MGRS is recognized as a premalignant condition, there is some evidence that some patients can benefit from treatments designed predominantly for hematologic malignancies.11
The 2012 IKMG guidelines recommend treatment of MGRS based on the underlying clone driving the pathophysiological process, advising consideration of age, clinical signs, patient comorbidities, renal metabolism, and nephrotoxicity of putative agents.4 At present, no evidence-based recommendations exist; only early phase clinical trial results and consensus opinion from expert groups are available.9 However, patients should always be treated upon progression to malignant disease, according to standard of care guidelines.6 Current examples of treatment for MGRS include:
- Bortezomib and dexamethasone for IgG, IgA, or light chain-producing plasma cell clones9
- Lenalidomide-cyclophosphamide-dexamethasone-based regimens for light chain amyloidosis12
- Bortezomib and melphalan with autologous stem cell transplant for monoclonal immunoglobulin deposition disease13,14
- Bortezomib and melphalan with autologous stem cell transplant for treatment of causal B-cell clones10
- Melphalan and dexamethasone for the treatment of early-stage light and heavy chain amyloidosis10
- Bortezomib, cyclophosphamide, and dexamethasone for Randall-type monoclonal immunoglobulin deposition disease10 and light chain amyloidosis9
- Rituximab-based therapy for lymphoplasmacytic clonal disease15
- Daratumumab for the treatment of proliferative glomerulonephritis with monoclonal Ig deposits and C3 glomerulopathy with MG16 and MGRS17
Where treatment is initiated, it has been shown that patients who achieve complete hematologic response to treatment preserve better renal function than those who only achieve partial response,16 with some patients demonstrating the potential for full renal recovery.13
Conclusion
This summary provides an overview of MGRS, the importance of the underlying pathological plasma cell clone identification, and the mechanisms of renal injury. Identifying disease-causing heavy and light chain antibodies can refine the diagnostic process, enabling both earlier diagnosis of MGRS and selection of appropriate treatment. Alongside more established treatments, daratumumab has demonstrated to be a safe and effective treatment option for MGRS in early phase clinical trials, with combination therapies showing preliminary deeper hematologic responses relative to daratumumab monotherapy. Large prospective multicenter studies are needed to inform evidence-based guidelines for the optimal treatment of patients with MGRS.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

