All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Longer term follow-up of the phase III trial SWOG S0777: VRd for patients with previously untreated MM
The Southwest Oncology Group (SWOG) S0777 trial (NCT00644228) was the first randomized, open label, phase III trial to evaluate the combination of bortezomib, lenalidomide plus dexamethasone (VRd) vs lenalidomide plus dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM). The published results in 2017 demonstrated a significant improvement in progression-free survival (PFS) and overall survival (OS) for the triple regiment combination compared with the doublet.1
This article summarizes the longer term outcomes with data cutoff of May 15, 2018, which was published by Brian GM Durie and colleagues in Blood Cancer Journal earlier this year.2
Study design and patient characteristics2
- 460 adult patients with NDMM were randomized 1:1 to receive VRd (n = 235) or Rd (n = 225)
- During induction, VRd was administered as eight 21-day cycles
- 1.3 mg/m2 bortezomib was administered intravenously on Days 1, 4, 8, and 11
- 25 mg lenalidomide was administered orally once daily on Days 1–14
- 20 mg dexamethasone was administered orally on Days 1, 2, 4, 5, 8, 9, 11, and 12
- During induction, Rd was administered as six 28-day cycles
- 25 mg lenalidomide was administered orally once daily for Days 1–21
- 40 mg dexamethasone was administered orally on Days 1, 8, 15, and 22
- All patients received 325 mg oral aspirin once daily to reduce the risk of thromboembolic complications
- Upon completion of induction, all patients received maintenance with Rd (25 mg oral lenalidomide once daily for 21 days plus 40 mg oral dexamethasone once daily for Days 1, 8, 15, and 22, of each 28-day cycle) until progressive disease, toxic effects, or patient withdrawal
- Primary endpoint: PFS
- Secondary endpoints: OS, overall response rate (≥ partial response), safety and to bank specimens for future translational medicine research
- Key inclusion criteria: presence of CRAB criteria with measurable and untreated disease
- Major exclusion criteria: creatinine clearance ≤ 30 mL/min; cardiac status New York Heart Association class III/IV or recent myocardial infarction; active hepatitis B or C or HIV or other uncontrolled infection; previous cancer prior to study registration or enrollment; or poorly controlled diabetes
- Patient characteristics can be seen in Table 1
- Characteristics were well balanced, although the Rd cohort had slightly more females, and those aged ≥ 65 years
Table 1. Baseline patient characteristics2
|
Hb, hemoglobin; ISS, International Staging System; LDH, lactate dehydrogenase; Rd, lenalidomide + dexamethasone; SWOG, Southwest Oncology Group; VRd, bortezomib + lenalidomide + dexamethasone |
||||
|
Characteristic |
All patients |
VRd |
Rd |
p value |
|
Age ≥ 65 years, % |
43 |
39 |
47 |
0.074 |
|
Female, % |
42 |
37 |
47 |
0.030 |
|
SWOG performance status > 1, % |
12 |
11 |
13 |
0.384 |
|
Creatinine ≥ 2 mg/dL, % |
5 |
5 |
4 |
1.000 |
|
LDH ≥ 190 U/L, % |
36 |
35 |
36 |
0.922 |
|
Hb < 10 g/dL, % |
32 |
33 |
31 |
0.764 |
|
Platelet count < 150 × 109/L, % |
17 |
15 |
20 |
0.176 |
|
ISS Stage III, % |
34 |
33 |
35 |
0.694 |
|
Intent to transplant, % |
68 |
69 |
68 |
0.841 |
Results2
- The median overall follow-up was 84 months with a median duration of maintenance of 17.1 months
- 53 patients were still on maintenance therapy at the time of data cutoff
- Outcome data can be seen in Table 2
- VRd outcomes were significantly better compared with Rd
- The primary report indicated a median OS of 75 months for VRd; with the update to patient follow-up and events, this is now not yet reached
Table 2. Outcome data2
|
CI, confidence interval; DOR, duration of response; HR, hazard ratio; NR, not reached; OS, overall survival; PFS, progression-free survival; Rd, lenalidomide + dexamethasone; VRd, bortezomib + lenalidomide + dexamethasone
|
||||
|
Outcome |
VRd |
Rd |
HR (95% CI) |
p value |
|
Median PFS, months |
41 |
29 |
0.742 (0.594, 0.928) |
0.0030 |
|
DOR, months |
50 |
39 |
|
0.0175 |
|
Median OS, months |
NR |
69 |
0.709 (0.543, 0.926) |
0.0114 |
|
Median OS by age, months |
|
|
|
|
|
< 65 years |
NR |
98 |
0.640 (0.421, 0.973) |
0.028 |
|
≥ 65 years |
65 |
56 |
0.769 (0.520, 1.138) |
0.168 |
|
5-year estimate of OS, % |
69 |
56 |
|
|
- Age-adjusted multivariable regression techniques were used to assess the impact of treatment within subgroups of interest including intent to transplant and age
- VRd achieved significantly longer PFS and OS in patients < 65 years, and in those who did not undergo a transplant or there was no intent to transplant
- Better, but not statistically significant, PFS and OS with VRd were also reported in patients presenting high-risk cytogenetics (t(14;14) and/or del(17p)), although additional data is needed to establish a clear benefit
- The trend favoring VRd over Rd was retained irrespective of age and intent to transplant classification
- Depth of best responses were assessed incorporating new serial data and additional bone marrow results, with a sensitivity analysis that included only assessable patients (Figure 1)
- ORR was 90.2% vs 78.8% for VRd and Rd, respectively
- Very good partial remission (VGPR) or better was 74.9% vs 53.2% for VRd and Rd, respectively
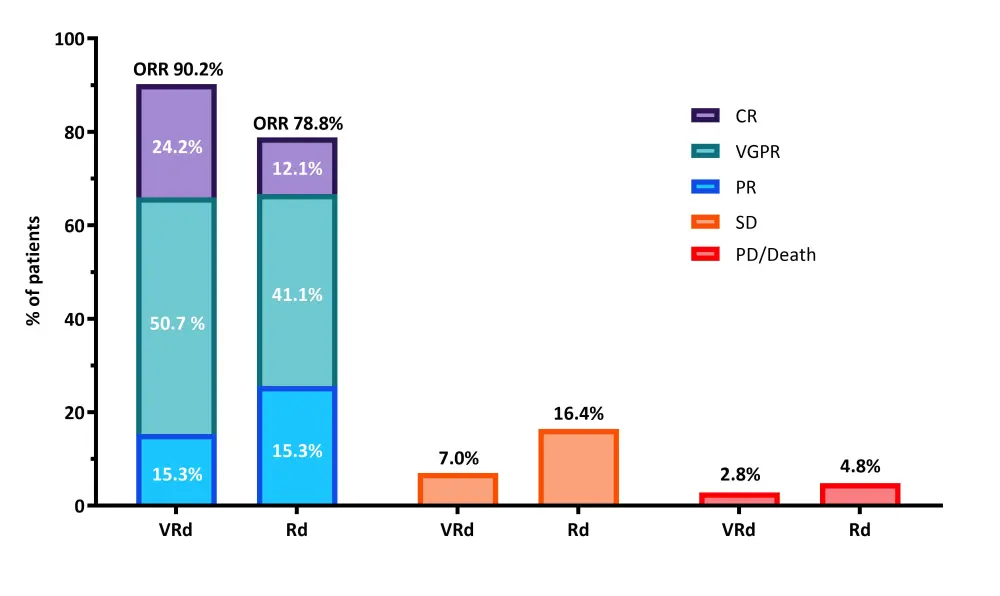
Figure 1. Best response in assessable patients
CR, complete response; ORR, overall response rate; PD, progressive disease; PR, partial response; Rd, lenalidomide + dexamethasone; SD, stable disease; VGPR, very good partial response; VRd, bortezomib + lenalidomide + dexamethasone
- Assessment of PFS (at 6 months) and OS (at 12 months) by best response revealed a longer PFS and OS for patients who achieved VGPR or better, compared with other responses
- Median PFS: > 38 months vs 10–16 months, respectively (p < 0.001)
- Median OS: > 76 months vs 38–50 months, respectively (p < 0.0001)
Treatment emergent adverse events and specific toxic effects were fairly well-balanced between the two treatment groups:
- Most common hematological AEs: lymphopenia, thrombocytopenia, anemia, neutropenia, and leukopenia.
- Overall, ~ 50% of patients experienced a Grade ≥ 3 hematological AE
- Most common nonhematological AEs: constitutional symptoms, infections, metabolic alterations, and neurologic events
- Grade ≥ 3 neurologic toxic effects (peripheral neuropathy) were significantly more frequent in the VRd cohort compared with the Rd cohort (34.6% vs 11.3%; p < 0.0001)
Conclusion
- With a follow-up of 7 years, there was still a statistically significant and clinically meaningful improvement in PFS and OS with VRd vs Rd induction therapy for patients with previously untreated MM
- VRd had acceptable safety and tolerability and continues to represent an appropriate standard of care, irrespective of age and transplant intent
- Treatment with VRd vs Rd induction led to deeper responses
- Limitations1,2 to the trial include:
- The use of intravenous bortezomib twice weekly resulted in neuropathy, which led to early discontinuations of the VRd induction therapy
- Lack of sufficient cytogenetic data to establish efficacy for high-risk patients
- The treatment cycles of VRd and Rd were not perfectly matched (eight 3-week cycles of VRd compared with six 4-week cycles of Rd) as this was the published protocol at the time the trial was initiated
- There was no PFS assessment for subsequent therapy response or remission duration
Earlier last year, the European Commission approved VRd for the treatment of multiple myeloma based on the results from this trial.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

