All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
KarMMa-3 trial: Updates from ASH 2023
Do you know... The final progression-free survival analysis of the KarMMa-3 trial showed a significantly higher overall response rate for ide-cel compared with standard regimens. What was the overall response rate for ide-cel?
Patients diagnosed with relapsed/refractory multiple myeloma (RRMM) who have been exposed to multiple prior lines of therapy have limited treatment options and a poor clinical prognosis. Idecabtagene vicleucel (ide-cel), a first-in-class chimeric antigen receptor T-cell therapy, has shown favorable responses and improved health-related quality of life (HRQoL) for the treatment of patients with triple-class exposed RRMM in the recent phase II KarMMa trial (NCT03361748).
During the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, updated results from the phase III KarMMa-3 trial (NCT03651128) investigating ide-cel compared with standard regimens in RRMM were presented. Updates focused on the final progression-free survival (PFS) analysis, real-world safety and efficacy, and HRQoL outcomes in patients who were diagnosed with triple-class exposed RRMM. We summarize key points below.
The full KarMMa-3 study design was previously reported by the Multiple Myeloma Hub.
Preplanned final PFS analysis from the KarMMa-3 trial1
- An additional 12.3 month follow-up period
- Primary endpoint was Independent Review Committee-assessed PFS in the intent-to-treat population
- Secondary endpoints were Independent Review Committee-assessed overall survival (OS) and overall response rate (ORR)
Results
- Median follow-up was 18.6 months
- Ide-cel showed a significant improvement in median PFS compared with standard regimens (13.3 months vs 4.4 months)
- 51% reduction in the risk of disease progression or death
- Other key response rates are shown in Figure 1
Figure 1. Key response rates from the KarMMa-3 trial at additional follow-up*
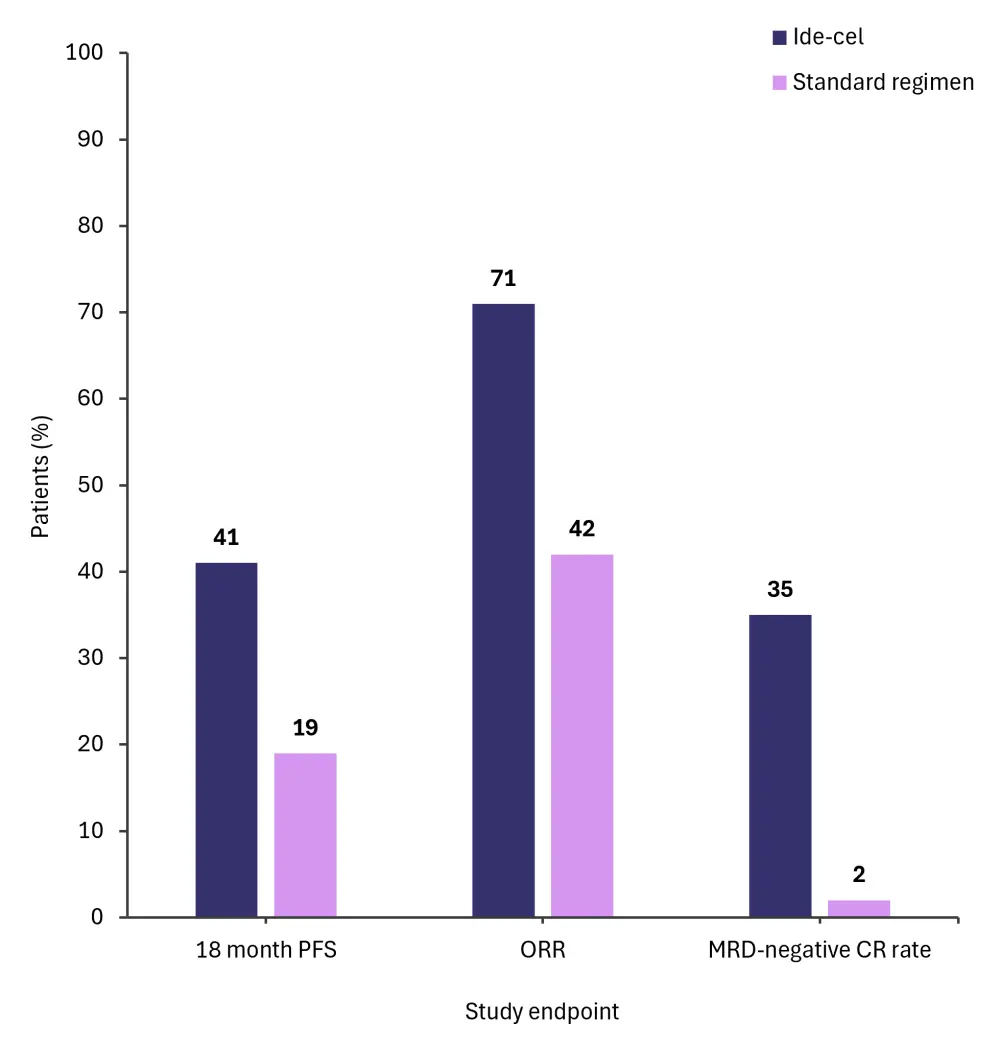
CR, complete response; ide-cel, idecabtagene vicleucel; MRD, measurable residual disease; ORR, overall response rate; PFS, progression-free survival.
*Adapted from Otero.1
- The median duration of response was longer in ide-cel treated patients compared with standard regimens (16.6 months vs 9.7 months)
- The median OS was also longer for ide-cel vs standard regimens in the intent-to-treat population (41.4 months vs 37.9 months)
- PFS and ORR results were consistent with those from the interim analysis
Safety
- No new safety signals were identified
- Cytokine release syndrome (CRS) of any grade was experienced by 88% of patients treated with ide-cel
- In total, 4% of patients experienced ≥Grade 3 CRS events
- Any-grade neurotoxicity was experienced by 15% of patients treated with ide-cel
- In total, 3% of patients experienced neurotoxic ≥Grade 3 events
Conclusion
- Patients treated with ide-cel experienced significantly longer PFS vs standard regimens
- The safety profile was manageable and consistent with previous analyses
- Results support the continued use of ide-cel in patients with triple-class exposed RRMM
Real-world efficacy and safety of ide-cel2
An analysis of 821 patients who received a commercial infusion of ide-cel.
- only 801 patients had PFS data
- median follow-up was 11.6 months
Efficacy
Response rates for the overall patient cohort are shown in Table 1.
Table 1. Real-world response rates for ide-cel*
|
CR, complete response; ide-cel, Idecabtagene vicleucel; ORR, overall response rate; PR, partial response; sCR, stringent CR; VGPR, |
|
|
Type of response, % |
Patients |
|---|---|
|
ORR |
73 |
|
≥VGPR |
56 |
|
CR/sCR |
25 |
|
VGPR |
31 |
|
PR |
17 |
- median PFS was 9 months
- median OS was not reached
- the 1-year OS estimate was 67%
- PFS for ide-cel in patient subgroups are shown in Table 2
Table 2. PFS for ide-cel in patient subgroups*
|
BCMA, B-cell maturation antigen; ide-cel, Idecabtagene vicleucel; PFS, progression-free survival. |
|
|
Patient subgroup, months |
PFS |
|---|---|
|
Cytogenetic risk |
|
|
High |
7.6 |
|
Standard |
9.74 |
|
Prior BCMA therapy |
|
|
<6 months |
4.9 |
|
≥6 months |
5.89 |
|
None |
9.67 |
|
Lymphodepletion type |
|
|
Bendamustine |
3.85 |
|
Fludarabine/cytarabine |
9.14 |
- OS was found to be inferior in patients treated with standard regimens compared with ide-cel in all subgroups (p = 0.019; p < 0.001; and p < 0.001, respectively)
Safety
- The real-world safety profile was as expected
- CRS of any grade was experienced by 80% of patients treated with ide-cel
- 3% of patients experienced a ≥Grade 3 CRS events
- Any-grade immune effector cell-associated neurotoxicity syndrome was experienced by 28% of patients treated with ide-cel
- Overall, 5% of patients experienced a Grade ≥3 immune effector cell-associated neurotoxicity syndrome event
- Infections were experienced by 45% of patients treated with ide-cel
- Prolonged cytopenia was experienced by 28% of patients treated with ide-cel
Conclusion
- This was the longest real-world study of ide-cel in patients with RRMM
- Efficacy and safety were both favorable, with an ORR of 73% and median PFS of 9 months
- This data supports the use of ide-cel in the heavily pretreated, real-world patient population.
HRQoL analysis from the KarMMa-3 trial3
- This analysis of HRQoL outcomes from the KarMMa-3 trial included an extended follow-up of patient-reported outcomes data.
- The primary outcome of this analysis was health status in several domains specified by the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire, including:
- physical and cognitive function;
- fatigue;
- pain;
- quality of life; and
- disease symptoms and side effects, as defined by the European Organization for Research and Treatment of Cancer Quality of Life Multiple Myeloma Questionnaire
Results
- The baseline patient-reported outcomes were comparable between the ide-cel group and standard regimen group
- Patients treated with ide-cel experienced significant and clinically meaningful improvements vs standard regimens in all the included domains
- Overall, the least square mean change from baseline to Month 25 showed significant improvements in patients treated with ide-cel vs standard regimen in 18 of 21 health domains
- The differences in these changes exceeded the threshold for the minimally important difference
- Time to confirmed deterioration was significantly longer for patients treated with ide-cel vs standard regimens in the following health domains:
- emotional;
- cognitive and social functioning;
- dyspnea; and
- constipation.
Conclusion
- Ide-cel significantly and meaningfully improved disease-associated symptoms compared with standard regimens
- Overall, 18 of 21 health domains showed statistical improvement, and 13 of 21 showed clinically meaningful improvement
- QoL-based improvements were experienced sooner with ide-cel treatment vs standard regimens and were sustained for >2 years
- Results showed extended HRQoL benefits with ide-cel vs standard regimens in patients with triple-class exposed RRMM
Overall conclusion
Collectively, the updated results from the phase III KarMMa-3 trial demonstrate ide-cel can significantly extend PFS compared with standard regimens, with a manageable safety profile in both clinical trials and real-world settings. Disease-associated symptoms and HRQoL were also significantly improved, supporting the continued use of ide-cel in heavily pretreated patient populations.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

