All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
KarMMa-3 trial of ide-cel vs SOC in RRMM meets its primary endpoint
On August 10, 2022, positive topline results were announced from the phase III KarMMa-3 trial (NCT03651128), which is comparing idecabtagene vicleucel (ide-cel) with standard combination regimens in patients with multiple myeloma that has been treated with two to four prior lines of therapy and is refractory to the last regimen.1
Following the prespecified interim analysis, it’s been established that the trial has met its primary endpoint of a statistically significant improvement in progression-free survival with ide-cel. Of note, this is the first randomized trial of a chimeric antigen receptor (CAR) T-cell therapy in multiple myeloma to communicate positive results. Further data from the KarMMa-3 trial will be released in a scientific conference soon.1
Idecabtagene vicleucel1
In March 2021, ide-cel became the first B-cell maturation antigen (BCMA)-targeted CAR T-cell therapy approved by the U.S. Food and Drug Administration (FDA) following data from the KarMMA trial. Similarly, ide-cel was granted a conditional marketing license by the European Commission (EC) in August 2021. Ide-cel is licensed for use after ≥4 prior lines of therapy by the FDA and ≥3 prior lines of therapy by the EC.
KarMMa-3 trial
The design of the KarMMa-3 trial, including inclusion/exclusion criteria and efficacy measures, can be seen Figure 1.
Figure 1. Design of the KarMMa-3 trial*
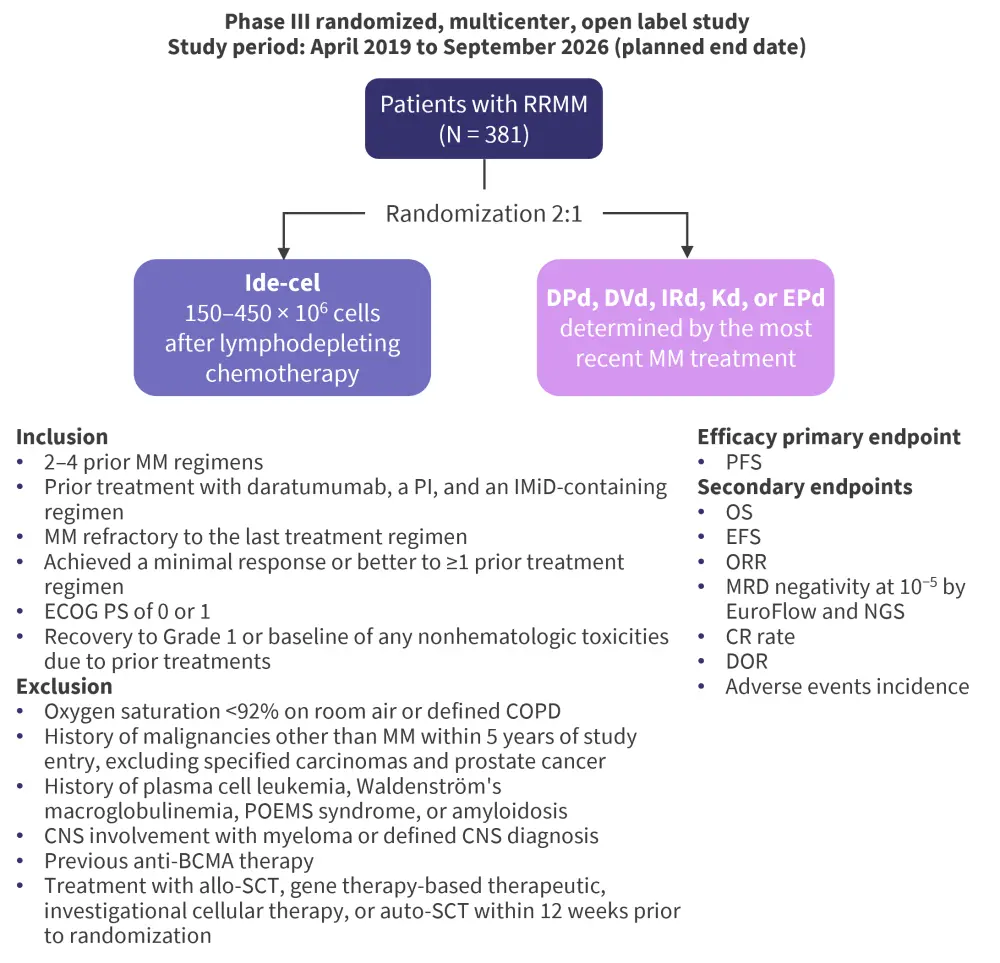
allo-SCT, allogeneic stem cell transplant; auto-SCT, autologous stem cell transplant; BCMA, B-cell maturation antigen; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; CR, complete remission; DOR, duration of response; DPd, daratumumab, pomalidomide, dexamethasone; DVd, daratumumab, bortezomib, dexamethasone; EPd, elotuzumab, pomalidomide, dexamethasone; ide-cel, idecabtagene vicleucel; IRd, ixazomib, lenalidomide, dexamethasone; ECOG, Eastern Cooperative Oncology Group; EFS, event-free survival; IMiD, immunomodulatory imide drug; Kd, carfilmozib, dexamethasone; MM, multiple myeloma; MRD, minimal residual disease; NGS, next-generation sequencing; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes; PS, performance status; RRMM, relapsed/refractory multiple myeloma.
*Data from ClinicalTrials.gov.2
Results1
This trial plans to recruit a total of 381 patients aged ≥18 years. The prespecified interim analysis demonstrated a statistically significant improvement in progression-free survival with ide-cel compared with traditional regimens, as well as improved overall response rates and overall survival rates. In addition, the safety and tolerability of ide-cel was reported to be consistent with prior clinical trial data. Further endpoint data are anticipated.
Of note, initial data from a multicenter study of ide-cel in a real-world setting were recently reported by Doris Hansen at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting. In the video below, Hansen discusses these initial data from a total of 159 patients.
Is experience with ide-cel CAR-T in the real-world setting comparable to the KarMMa trial results?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



