All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Incidence of cytopenias and infections in patients with R/R MM after treatment with idecabtagene vicleucel
Idecabtagene vicleucel (ide-cel) was approved for use in patients with relapsed/refractory (R/R) multiple myeloma in March 2021, following results from the pivotal phase II KarMMa trial (NCT03361748). Cytopenias and infections were common in enrolled patients, with Grade ≥3 cytopenias commonly occurring in the first 8 weeks posttreatment.
Below, we present data from a multi-center retrospective study that evaluated the occurrence of cytopenias and infections in patients receiving ide-cel in a standard of care (SOC) setting and the need for supportive therapies required posttreatment in these patients.
Study design
A total of 52 adult patients with R/R MM who received SOC ide-cel treatment between May 1 and December 30, 2021, were included in this study. Patients underwent apheresis as per institutional SOC, then were given fludarabine/cyclophosphamide lymphodepletion, followed by SOC ide-cel infusion. Cytopenias and infections were measured up to Day 100, after which data was censored. Data was censored before 100 days if patient death or progressive disease occurred. A total of 47 patients reached day 90 follow-up.
Patient characteristics
Patient baseline characteristics are given in Table 1.
Table 1. Patient characteristics at baseline*
|
ECOG, Eastern Cooperative Oncology Group. |
|
|
Characteristic, % (unless otherwise stated) |
Patients (N = 52) |
|---|---|
|
Median age (range), years |
66 (43–78) |
|
Male |
44 |
|
ECOG Performance Status pre-lymphodepletion |
|
|
0 |
27 |
|
1, 2, or 3 |
73 |
|
High-risk cytogenetics |
40 |
|
Median prior lines of therapy (range), n |
6 (4–13) |
|
Triple-refractory disease† |
87 |
|
Penta-refractory disease‡ |
44 |
|
Extramedullary disease |
62 |
Results
Safety and efficacy
Overall, 85% of all patients experienced any grade cytokine release syndrome (CRS), with 6% of patients experiencing Grade ≥3. Any grade and Grade ≥3 immune effector cell-associated neurotoxicity syndrome (ICANS) was experienced by 19% and 8% of patients, respectively. Of the 52 patients included in the study, 51 patients reached Day 30, one patient died due to Grade 5 CRS. At Day 90, 47 patients were evaluable, one further patient died from neurological toxicity, and three patients had progressive disease. Partial response or better was achieved in 92% of patients in the first 90 days, of which 48% achieved a complete response (CR) or stringent complete response (sCR).
Occurrence of cytopenias
Any grade cytopenias were common at apheresis, occurring in 92% of patients, and increasing to 100% of patients at Day 0. Figure 1 and Figure 2 illustrate the incidence of cytopenias of any grade, and Grade ≥3 from apheresis until Day 90. Univariate analyses identified that occurrence of Grade ≥3 cytopenias at Day 30 after ide-cel treatment was associated with several risk factors, including female sex, prior-BCMA-directed therapy, longer time from last bridging therapy to lymphodepletion (median 29 days and 15 days for patients with Grade ≥3 cytopenia at Day 30 and those without, respectively), presence of any grade anemia pre-lymphodepletion, and presence of circulating plasma cells pre-lymphodepletion. However, multivariable analysis showed no link between these risk factors and Grade ≥3 cytopenias at Day 30. Power to detect an association may be limited due to the relatively small sample size.
Figure 1. Incidence of any-grade cytopenias from apheresis to Day 90*
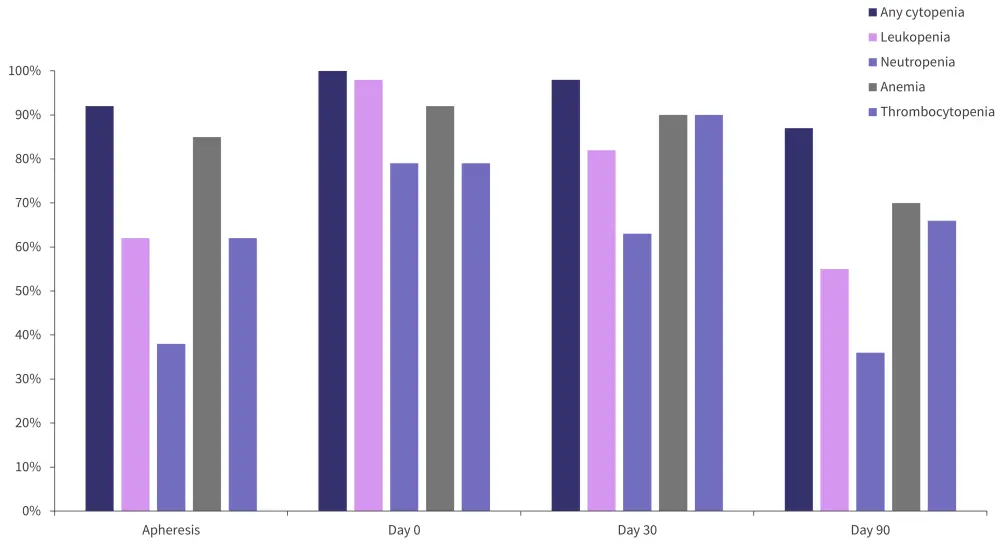
*Adapted from Logue, et al.1
Figure 2. Incidence of Grade ≥3 cytopenias from apheresis to Day 90*
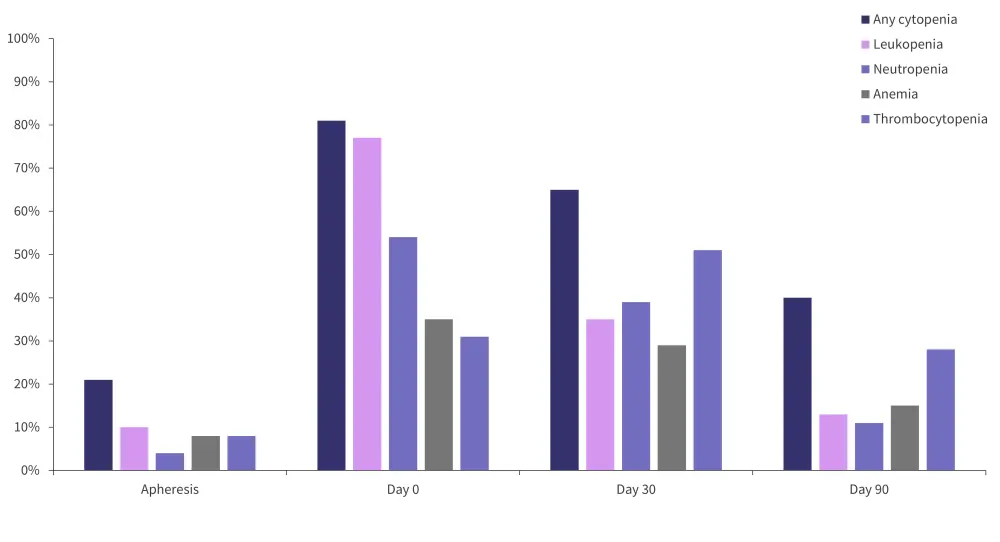
*Adapted from Logue, et al.1
Occurrence of infections
In the first 100 days after ide-cel treatment, 54% of patients experienced infections (23% of which were severe); 68% were bacterial from Days 31 to 100 and 50% and 42% were bacterial and viral, respectively, from Days 31 to 100. In univariate analyses, a longer time from the last bridging therapy to lymphodepletion was the only factor which was significantly associated with risk of infection. However, no statistically significant association was found between severe infection risk and treatment-related factors.
Supportive therapies
All supportive therapies were given at the discretion of the treating physician. In the first 100 days after ide-cel infusion, 65% of patients required transfusion, 52% of which were transfused packed red blood cells. No patients required erythropoiesis-stimulating agents. A total of 88% of patients received granulocyte-colony stimulating factor and 21% of patients received thrombopoietin (TPO) receptor agonist; 19% of all patients remained thrombocytopenic and continued on thrombopoietin agonists at Day 100.
Conclusion
As observed in the KarMMa trial, this study reported high incidence of severe cytopenias, and similar incidence of immune effector cell-associated neurotoxicity syndrome, and Grade ≥3 CRS. Incidence of infection and cytopenia in patients with R/R multiple myeloma treated with ide-cel is high, requiring supportive care and management for potentially life-threatening toxicities. The management of CAR-T cell complications has been previously reported on the Multiple Myeloma Hub. Potential risk factors for Grade ≥3 cytopenia were identified by univariable analysis, although none were identified by multivariable analysis potentially due to the small sample size. Further studies including a larger sample size and a longer follow-up in this patient population could confirm if these risk factors are significant and require specialized management approaches. However, despite cytopenia and infection presenting complications following treatment with SOC ide-cel, disease progression remains the key challenge for patients with R/R multiple myeloma.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

