All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Management of short-, medium-, and long-term complications in patients receiving CAR T-cell therapy
Do you know... Cytokine release syndrome is a frequent short-term complication observed during CAR T-cell therapy. It occurs 1–14 days post infusion and is the result of effector cytokine activity triggering further proinflammatory cytokine release. Which next treatment is advised if cytokine release syndrome is not controlled after two doses of tocilizumab?
Chimeric antigen receptor (CAR) T-cell therapies are widely used to treat relapsed/refractory (R/R) B-cell malignancies, with four CAR T-cell products currently approved in Europe: tisagenlecleucel for pediatric B-cell acute lymphoblastic leukemia (B-ALL) and adult large B-cell lymphoma (LBCL), axicabtagene ciloleucel for adult LBCL, KTE-X19 for adult mantle cell lymphoma, and idecabtagene vicleucel for adult multiple myeloma. CAR T-cell therapy is associated with potentially life-threatening immunological toxicities. As such, comprehensive training for personnel involved in its delivery, including intensive care unit and neurology specialists, is vital.1
In 2021, the European Group for Blood and Marrow Transplantation (EBMT) and the European Hematology Association (EHA) proposed an expanded revision of the 2019 EBMT-Joint Accreditation Committee of ISCT and EBMT (JACIE) guidelines for the use of CAR T-cell therapy.1 This involved 36 experts drafting recommendations based on the current literature to ensure continued best practice. Due to the absence of randomized trial evidence, the recommendations are not graded but are a consensus view of the authors. Here, we summarize the guidelines for the management of short-, medium-, and long-term complications associated with CAR T-cell therapy.1
Management of short-term complications
Short-term complications are conditions experienced from the day of admission to Day 28 post therapy.
Tumor lysis syndrome
Tumor lysis syndrome may be seen in some patients treated with CAR T cells and should be managed according to standard local protocols.
Infection
Active infections must be controlled before treatment initiation, as all patients will be neutropenic once infusion begins. Signs of fever should be dealt with as follows:
- prompt empirical antimicrobial therapy
- blood and urine cultures
- chest x-ray and/or high-resolution computed tomography (CT) of the chest
Respiratory viral screening is also advised, including for COVID-19. In selected cases, a lumbar puncture and brain magnetic resonance imaging (MRI) may also be suitable: the risk profile of the patient should guide diagnostic work-up and antimicrobial selection.
Cytokine release syndrome
Cytokine release syndrome (CRS) occurs in 30–100% of patients and is a Grade ≥3 event in 10–30% of patients. The onset of the condition usually occurs 1–14 days post infusion and typically lasts for 1–10 days. Symptoms are as follows:
- fever ≥38°C
- hemodynamic instability
- hypoxemia
CRS is caused by effector cytokine release, such as IL-2, triggering further release of pro-inflammatory cytokines, including IL-6 and monocyte chemoattractant protein-1. The suggested management algorithm is shown in Figure 1. When two doses of tocilizumab fail to control CRS, dexamethasone is advised. If the CRS is not controlled by tocilizumab or steroids, other therapeutic options include siltuximab and anakinra; however, as limited clinical data are available, these options should only be considered if there is a high suspicion of underlying infection or macrophage activation syndrome (MAS).
Figure 1. Management of CRS*
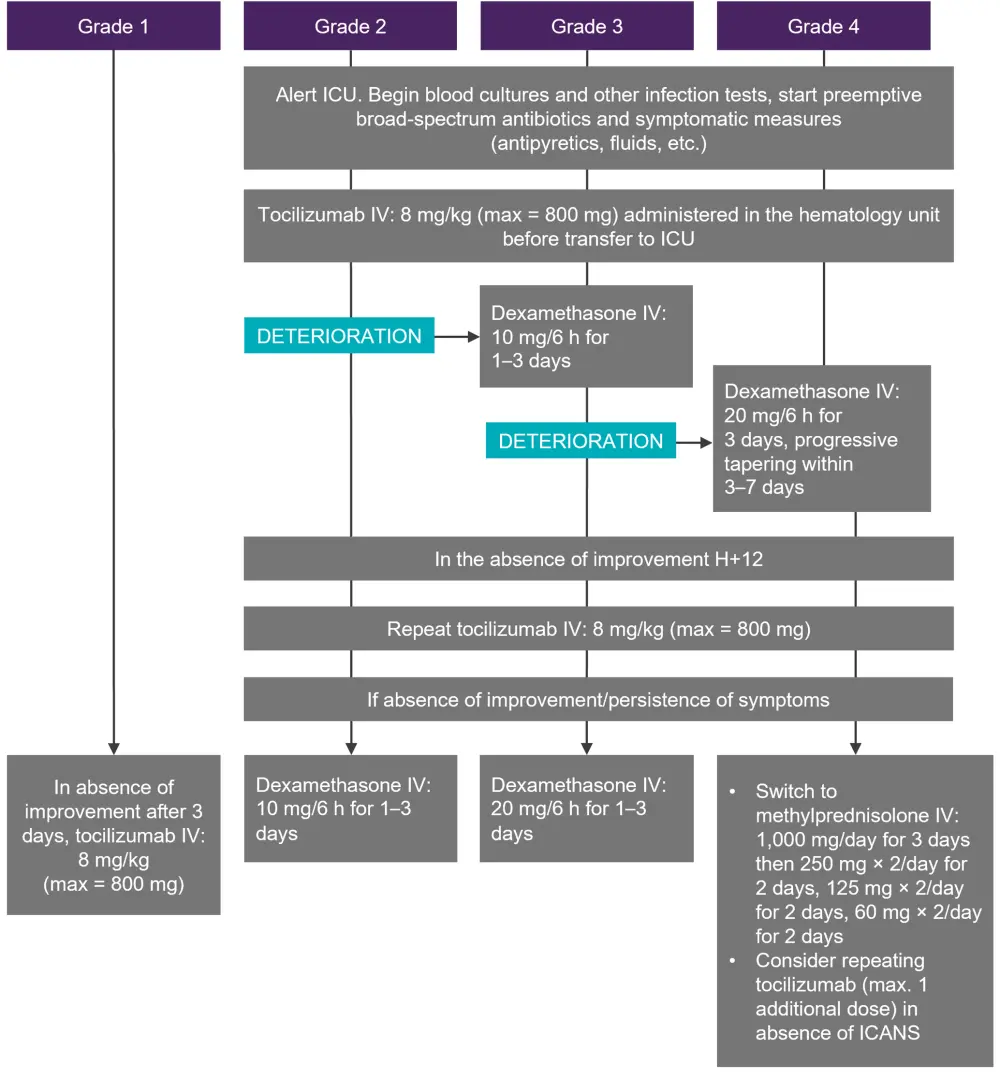
CRS, cytokine release syndrome; h, hour; ICU, intensive care unit; IV, intravenous.
*Adapted from Hayden, et al.1
Macrophage activation syndrome
MAS is identified as persistent fever with organomegaly, cytopenias, hyperferritinemia (>10,000 ng/ml), liver disfunction, coagulopathy, and hypertriglyceridemia. The suggested management algorithm for MAS and CRS overlap is shown in Figure 2. If the patient has refractory CRS or MAS, chemotherapy may be considered, although this poses the risk of destroying the CAR T cells.
Figure 2. Management of CRS and MAS*
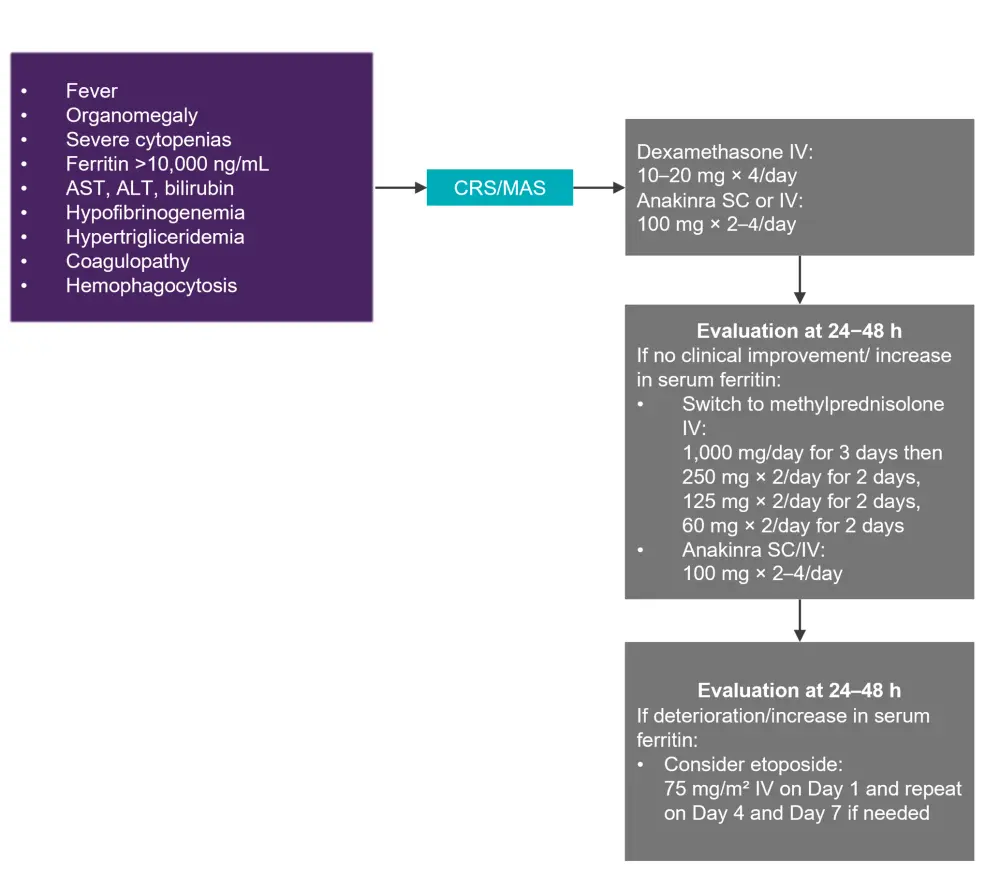
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRS, cytokine release syndrome; IV, intravenous; MAS, macrophage activation syndrome; SC, subcutaneous.
*Adapted from Hayden, et al.1
Immune effector cell-associated neurotoxicity syndrome
Immune effector cell-associated neurotoxicity syndrome (ICANS) affects 20–60% of patients treated with CD19 CAR T cells, typically 3–5 days post CAR T-cell infusion, with 10% experiencing a delay of >3 weeks. ICANS is also seen, albeit to a lesser extent, with B-cell maturation antigen-targeted CAR T-cell treatment. The syndrome is likely to be caused by inflammatory cytokines increasing vascular permeability and endothelial activity leading to the breakdown of the blood–brain barrier and an increase in cerebral spinal fluid cytokines. Symptoms are as follows:
- tremors
- confusion
- agitation
- seizures
Diagnosis is achieved through a CT of the head, clotting screen/fibrinogen, and electroencephalogram (EEG), MRI, and a lumbar puncture in severe, or steroid-refractory cases. The suggested treatment algorithm for ICANS is shown in Figure 3.
Figure 3. Management of ICANS*
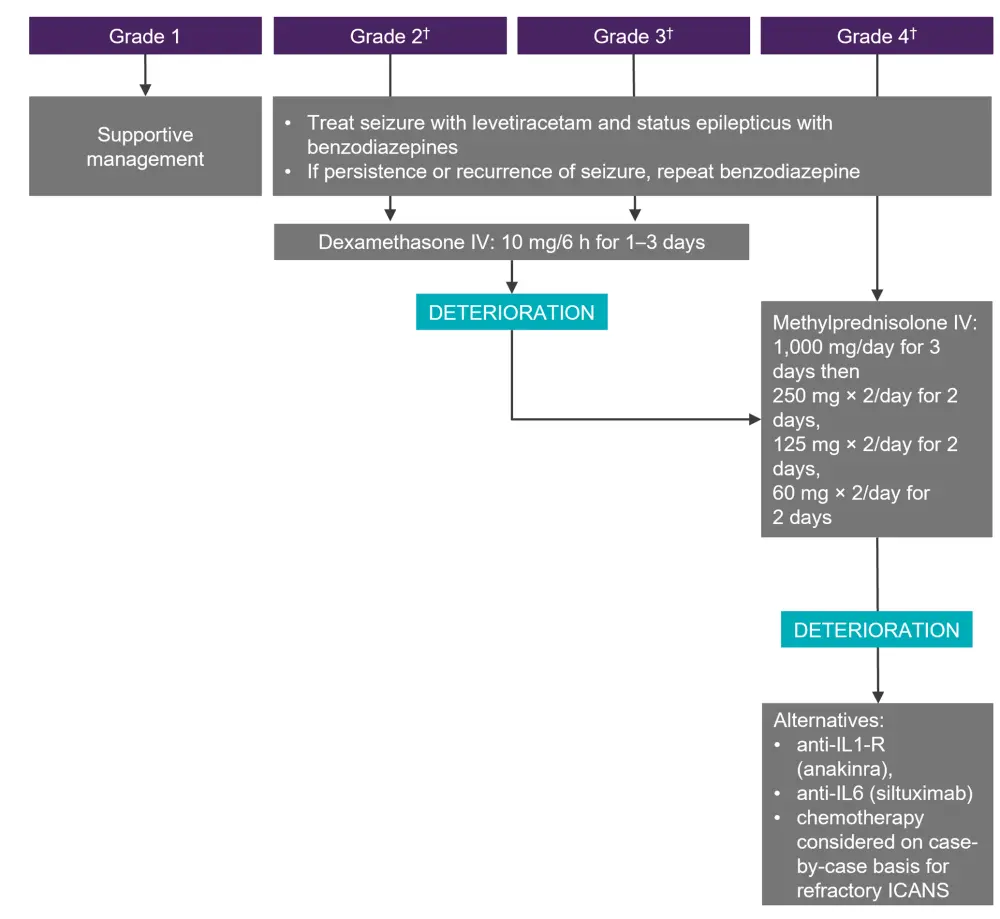
CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; IV, intravenous.
*Adapted from Hayden, et al.1
†In the setting of Grade ≥2 ICANS with concurrent Grade 1 CRS, steroids should be administered (not tocilizumab). This does not apply for higher-grade CRS.
Cardiovascular toxicity
Cardiovascular toxicity occurs in 10–20% of patients and is an early and largely reversible phenomenon. Hypertension was the main complication in early pediatric studies, requiring vasopressor support. Other symptoms include the following:
- arrythmias
- myocardial impairment
- left ventricular systolic dysfunction
- cardiac failure
Thorough cardiovascular assessments pre-CAR T-cell infusion with surveillance and risk reductions may reduce complications. Elevated baseline serum cardiac biomarkers may signal a greater risk of CAR T-cell infusion-related cardiotoxicity. Management is otherwise through an electrocardiograph (ECG) to exclude underlying arrythmias, transthoracic echocardiography to define the baseline left ventricular ejection fraction, and diastolic function to predict left ventricular systolic dysfunction. Tocilizumab has also been associated with rapid improvement in complications.
Management of medium-term complications
Although rare, medium-term complications (between Day 28 and Day 100 post infusion) of tumor lysis syndrome, CRS, and ICANS can occur and should be managed according to the guidance outlined above.
Infection and prophylaxis
Opportunistic infections are common; therefore, antimicrobial prophylaxis should be administered until immune reconstitution. Most infections within the first 30 days of treatment are bacterial and respiratory viral. However, beyond Day 30, viral infections are the most common. Although uncommon, reactivation is possible for the following:
- herpes simplex virus (HSV)
- varicella-zoster virus
The following viral infections are considered rare but can also be problematic if reactivated:
- cytomegalovirus (CMV)
- Epstein–Barr virus (EBV)
- adenovirus
- human herpesvirus 6
- BK polyomavirus
- John Cunningham virus
COVID-19 currently presents the most difficult problem. For patients with hepatitis B virus, hepatitis C virus and HIV infection, CAR T-cell therapy is safe provided the virus is undetectable. For hepatitis B infection, long-term entecavir, tenofovir, or equivalent prophylaxis is also recommended.
B-cell aplasia
B-cell aplasia is associated with sinopulmonary infections and should have consistent monitoring. Immunoglobin replacement is routine in pediatric CAR T-cell therapy. Long-lived plasma cells may confer a protective effect in adults; however, immunoglobulin replacement is still preferred to replacement therapy. Replacement therapy aims to maintain serum levels >4 g/l in adults and within age-adapted normal ranges for pediatric patients. Replacement therapy should be continued until recovery of functional B cells.
Vaccination
Incomplete immune reconstitution and ongoing immune suppression is associated with a high chance of a lower vaccine response, including for COVID-19. However, the agreed view is that vaccination may reduce infection rates and improve clinical outcomes. Laboratory assessments of cellular and humoral immunity should be performed where available, along with specific antibody responses.
In the following interview, Arnon Nagler (Sheba Medical Center, Tel Aviv, IL) explains the importance of vaccination against COVID-19 for patients receiving treatment with CAR T cells, but stresses that vaccination should be administered ≥6 months after CAR T-cell treatment.
How do CAR T-cell recipients respond to COVID-19 vaccination?
Graft-versus-host disease
Patients post allogeneic hematopoietic stem cell transplantation are generally considered safe to undergo CAR T-cell therapy. However, diagnosis and management of graft-versus-host disease should be performed according to standard protocols. Clinicians must also balance the benefits of systemic immunosuppression against the adverse impact of CAR T-cell viability.
Prolonged cytopenias
Hematologic toxicity has a cumulative 1-year incidence of 58% post CAR T-cell therapy and is often prolonged, following a two-phase course with initial neutrophil recovery followed by a second fall. The duration and severity of the condition differs between CAR T-cell products, with symptoms as follows:
- persistent Grade ≥3 neutropenia
- thrombocytopenia
- anemia after Day 28
Granulocyte colony-stimulating factor is recommended for severe neutropenia (<0.5 × 109/L) from Day 14. Patients who are refractory will have a neutrophil count <100/µl lasting ≥30 days, conferring a risk of fungal infection. Further treatment options are as follows:
- autologous stem cell rescue
- unconditioned CD34+ cells—supplemented for patients post allogeneic hematopoietic cell transplantation
- Dexamethasone and erythropoietin/thrombopoietin agonists
Management of long-term complications
Long-term complications arise after Day 100 post CAR T-cell infusion. As such, patient follow-ups should be conducted by multidisciplinary teams to analyze disease status and late effects. Prolonged cytopenias, hypogammaglobulinemia, and infections are common, while neurological complications and pulmonary toxicity are associated with an increased mortality risk. Secondary malignancies are rare, with only a single case reported following transduction of a leukemic B cell during manufacturing. A recent publication described CAR T-cell-derived malignancies following genome-edited CAR T-cell therapy due to insertional mutagenesis.2
Conclusion
Complications can arise at any time after the administration of CAR T-cell therapy; however, with these updated guidelines, multidisciplinary teams should be able to effectively manage these, enabling the best treatment outcomes for patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




