All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Does current reporting on CAR T-cell therapy in clinical trials overestimate efficacy? Why the distinction between ITT and mITT populations is important
Anti-CD19 chimeric antigen receptor (CAR) T-cell therapy has resulted in impressive and durable remissions in patients with relapsed/refractory acute lymphoblastic leukemia (ALL) and B-cell lymphomas, and anti-B-cell maturation antigen (BCMA) CAR T-cell therapy has shown promise for heavily pretreated patients with multiple myeloma (MM). While this therapy has changed—and continues to change—the treatment landscape for these hematologic malignancies, it is not without challenges.1
Access to CAR T-cell therapy is often dependent on proximity to treatment centers that have the capability to administer it; CAR T-cell therapy is administered at a limited number of cancer centers and is generally delivered in an inpatient setting, which contributes to the already considerable cost associated with this therapy.2 Even if a patient is fortunate enough to have access to CAR T-cell therapy, there are often delays in collection and production during which disease progression can (and often does) occur. These patients are then excluded from efficacy analyses, creating a selection bias in which efficacy is reported for only a modified subset of patients with less aggressive disease. Mohyuddin et al.performed a literature review and meta-analysis regarding this selection bias, which we have summarized here.1
Study design
Methods
Mohyuddin et al. performed a systematic review of CD19 and BCMA-targeting CAR T-cell therapy trials after searching queries in four databases: Web of Science, MEDLINE/PubMed, EMBASE, and Cochrane Registry of Controlled Trials. All prospective trials that enrolled at least two patients and were published between January 1, 2013, and November 1, 2020, were included. For CD19, all leukemia and lymphoma subtypes were included. Editorials, case reports, case series, and review articles were excluded.
Outcomes
The primary outcome was the proportion of trials reporting the number of patients enrolled and the number of patients who actually received CAR T-cell therapy. Secondary outcomes, which were calculated for trials that met the primary outcome, included the overall response rates (ORRs) on an intent-to-treat (ITT) analysis incorporating all enrolled patients. It should be noted that the ORRs were defined by their respective studies and therefore varied based on the disease: for MM trials, these were largely based on response criteria from the International Myeloma Working Group, and for lymphoma trials, the Lugano criteria.
Results
Multiple myeloma: BCMA CAR T-cell therapy studies
Of the 346 records identified through database searches, 28 BCMA CAR T-cell therapy clinical trials for patients with MM met the inclusion criteria; ten of these trials reported the number of patients enrolled and the number of patients who received CAR T-cell therapy (Figure 1).
Figure 1. Study selection for BCMA CAR T-cell therapy studies*
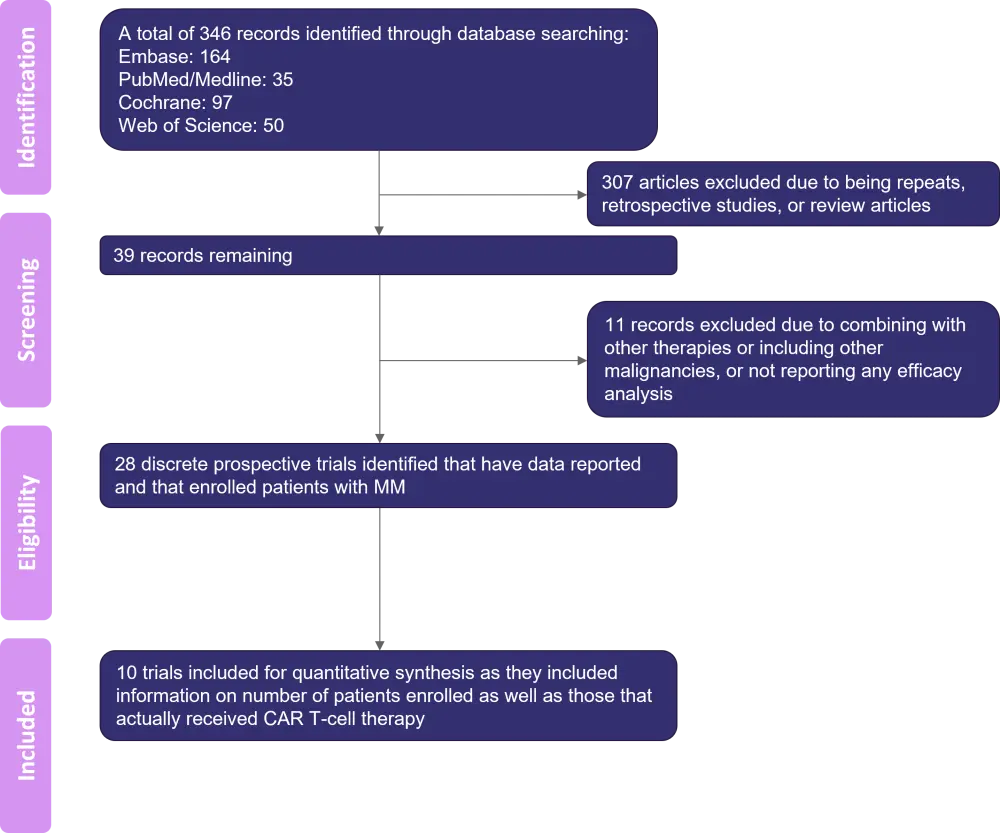
BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; MM, multiple myeloma.
*Adapted from Mohyuddin, et al.1
Regarding these ten studies, the pooled ORR in the modified ITT (mITT) population was 78.0% (95% confidence interval [CI], 67.0–89.0%; I2 = 87.90%). An analysis of the ITT population—which included all patients, even those who were enrolled but did not subsequently receive CAR T-cell therapy—revealed a pooled ORR of 70% (95% CI, 59.0–80.0%; I2 = 80.6%). A total of 395 patients were enrolled and received CAR T-cell therapy, with efficacy results reported, while 46 patients did not receive CAR T-cell therapy after enrolling. For 6 (3.1%) of these patients, rapid disease progression necessitating alternate therapy was the reason for not receiving CAR T-cell therapy. For 40 (86.9%) of these patients, the reasons for not receiving CAR T-cell therapy were not reported.
For all 28 BCMA trials meeting the enrollment criteria, an additional 47 patients were excluded from efficacy analysis despite having received CAR T-cell therapy:
- One patient (2.2%) was excluded due to early death from infection.
- The remaining 46 patients (97.8%) were excluded due to a follow-up that was insufficient to assess for efficacy.
CD19 CAR T-cell therapy studies
There were 74 CC19 CAR T-cell therapy studies identified, 52 (70.2%) of which reported the total number of patients who were enrolled and the number of patients who received CAR T-cell therapy (Figure 2).
Figure 2. Study selection for CD19 CAR T-cell therapy studies*
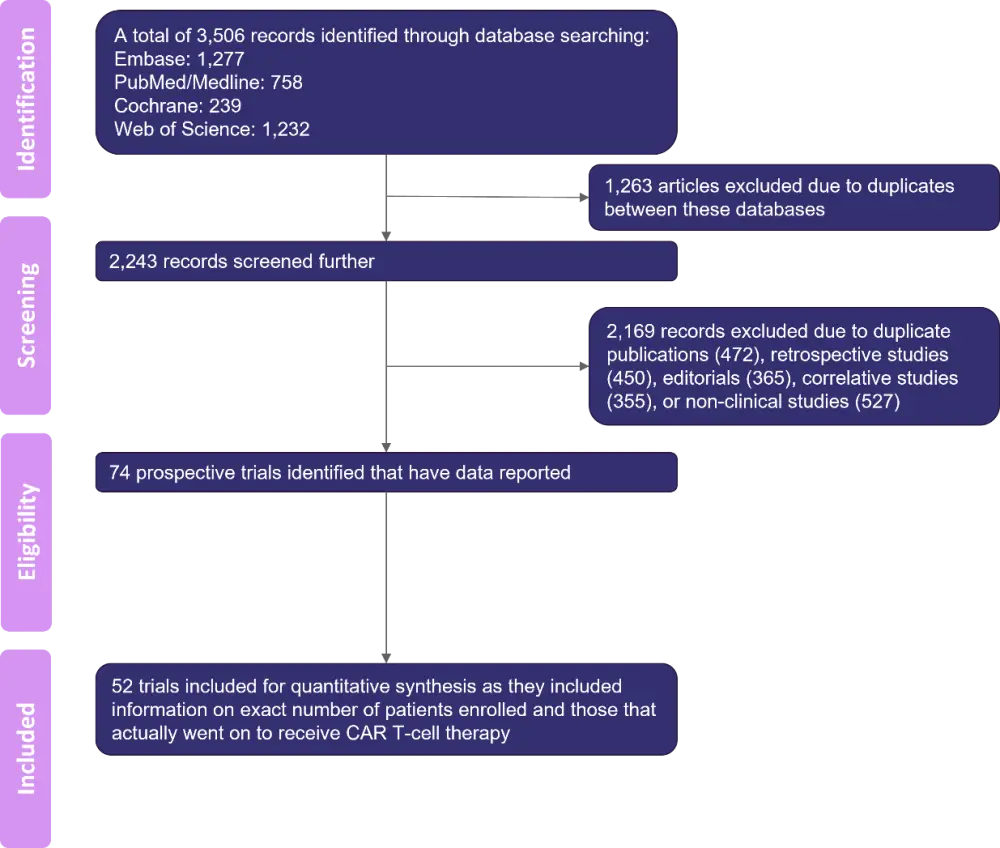
CAR, chimeric antigen receptor.
*Adapted from Mohyuddin, et al.1
The CD19 studies were stratified into those that exclusively studied leukemia or lymphoma. There were 26 studies that exclusively enrolled patients with leukemia, with a pooled ORR in the mITT population of 87.2% with moderate heterogeneity (95% CI, 83.1–91.2%; I2 = 56.6%). The pooled ORR in the ITT analysis was 74.9% with high heterogeneity (95% CI, 64.8–85.0%; I2 = 92.3%). There were 14 studies that exclusively enrolled patients with lymphoma, with a pooled ORR in the mITT population of 70.7% with moderate heterogeneity (95% CI, 63.9–77.5%; I2 = 54.7%). Analysis of the ITT population revealed a pooled ORR of 58.7% with high heterogeneity (95% CI, 49.7–67.7%; I2 = 92.13%).
For all 52 CD19 studies—including those that enrolled multiple CD19 malignancies—the pooled ORR for the mITT analysis was 79.0% (95% CI, 74.8–83.2%, I2 = 73.9%), while the pooled ORR for the ITT analysis was 68.1% (95% CI, 61.3–74.8; I2 = 90.3%). Among these 52 studies, 28 trials enrolled patients who did not subsequently receive CAR T-cell therapy. In these 28 trials, there were 266 patients who did not receive CAR T-cell therapy after enrolling, and 113 were excluded from efficacy analyses despite having received CAR T-cell therapy (Table 1).
Table 1. Reasons for not receiving CAR T-cell therapy or for exclusion from efficacy analyses, CD19 CAR T-cell therapy trials*
|
CAR, chimeric antigen receptor; MRD, minimal residual disease; PET, positron emission tomography. |
|
|
Reasons, n (%) |
|
|---|---|
|
Did not receive CAR T-cell therapy after enrollment (n = 266) |
|
|
Not reported or other reasons |
121 (45) |
|
Death |
49 (18) |
|
Difficulties with manufacturing CAR T-cell therapy |
22 (8) |
|
Response to prior therapy/conditioning rendering them ineligible for CAR T-cell therapy |
22 (8) |
|
Disease progression or disease-related complications |
21 (8) |
|
Infection |
10 (8) |
|
Insufficient follow-up at time of analysis |
(5.2) |
|
Received CAR T-cell therapy but were excluded from efficacy analyses (n = 166) |
|
|
Not reported/other |
35 (31) |
|
Not yet evaluable for response |
29 (26) |
|
Received nonconforming product |
25 (22.1) |
|
Death |
(9.8) |
|
Inability to obtain PET scan before treatment |
6 (5) |
|
Achievement of MRD negativity/PET response before administration of product |
5 (4) |
|
CAR T-cell therapy given at a greater than maximum dose |
2 (2) |
|
Lost to follow-up |
2 (2) |
Sensitivity analysis revealed a slight small study bias (Begg statistic p = 0.037), and most studies had similar precision. When single studies were omitted, no study was shown to have a significant influence on the overall results, and this was true for both BCMA and CD19 CAR T-cell therapy.
Conclusion
It is important to note that CAR T-cell therapy has ushered a paradigm shift in the treatment of patients with hematologic malignancies: prolonged and durable remissions have been elicited in patients, particularly those who have received CD19-targeted CAR T-cell therapy, and the real world efficacy of axicabtagene ciloleucel has been in line with the efficacy reported in clinical trials. The results reported here are also limited due to small sample sizes, as well as significant heterogeneity between studies, which was higher in the ITT analyses compared with the mITT analyses. However, this meta-analysis shows that CAR T-cell therapy trials are indeed susceptible to selection bias, with differences in response rates between ITT and mITT analyses ranging from 8–12%. Prospective collection of real world data and transparent reporting of the number of patients who are unable to receive CAR T-cell therapy after enrollment (including reasons for this) are essential to understand the true efficacy of CAR T-cell therapy and correctly identify patients who are eligible for and will benefit from this therapy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

