All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Impact of treatment modality and route of administration on rates of CRS in RRMM
B-cell maturation antigen (BCMA)-directed immunotherapies for the treatment of relapsed/refractory multiple myeloma (RRMM) are often associated with high response rates compared with standard of care treatments. However, they are often also accompanied by cytokine elevation, which can lead to cytokine release syndrome (CRS). The incidence of CRS associated with BCMA-directed therapies is well established but rarely stratified by modality and administration route.1
Here, we summarize a meta-analysis by Soltantabar et al.1 published in Clinical Pharmacology and Therapeutics on the impact of treatment modality and the route of administration of immunotherapies on the incidence of CRS in RRMM.
Study design1
- In this meta-analysis, data were collected on the incidence of CRS from trials including patients with RRMM treated with BCMA-directed chimeric antigen receptor (CAR) T-cell or bispecific antibody (bsAb) therapies.
- The weighted proportion of Grade ≥3 CRS events were compared by modality of treatment and administration (intravenous/subcutaneous).
- Data were also collected on the rate and agent used to manage CRS, as well as onset and duration.
Key findings1
- In total, 1,560 patients enrolled across 36 studies were included in this study.
- A higher incidence of CRS was associated with the use of CAR T-cell therapies compared with bsAbs, in all grades of CRS (Figure 1).
- Rates of CRS also varied significantly by dose level per agent (Table 1).
- CAR T-cell therapies were also associated with a longer median duration of CRS at 5 days vs 2 days with bsAbs.
- The incidence of any-grade CRS following treatment with BsAbs was comparable between intravenous and subcutaneous administration routes at 58% vs 59%, respectively.
- However, a lower incidence of Grade ≥3 CRS was observed in patients who received subcutaneous bsAbs at 0% vs 4% intravenous.
- The number of studies investigating the subcutaneous administration route was limited; therefore, more research is needed to draw conclusions.
- Tocilizumab and corticosteroids were more commonly used to manage CRS in patients treated with CAR T-cell therapies than bsAbs.
- CAR T-cell therapy: 44% and 13%, respectively.
- BsAb therapy: 25% and 13%, respectively.
Figure 1. CRS rates associated with CAR T-cell and bispecific antibody therapies*
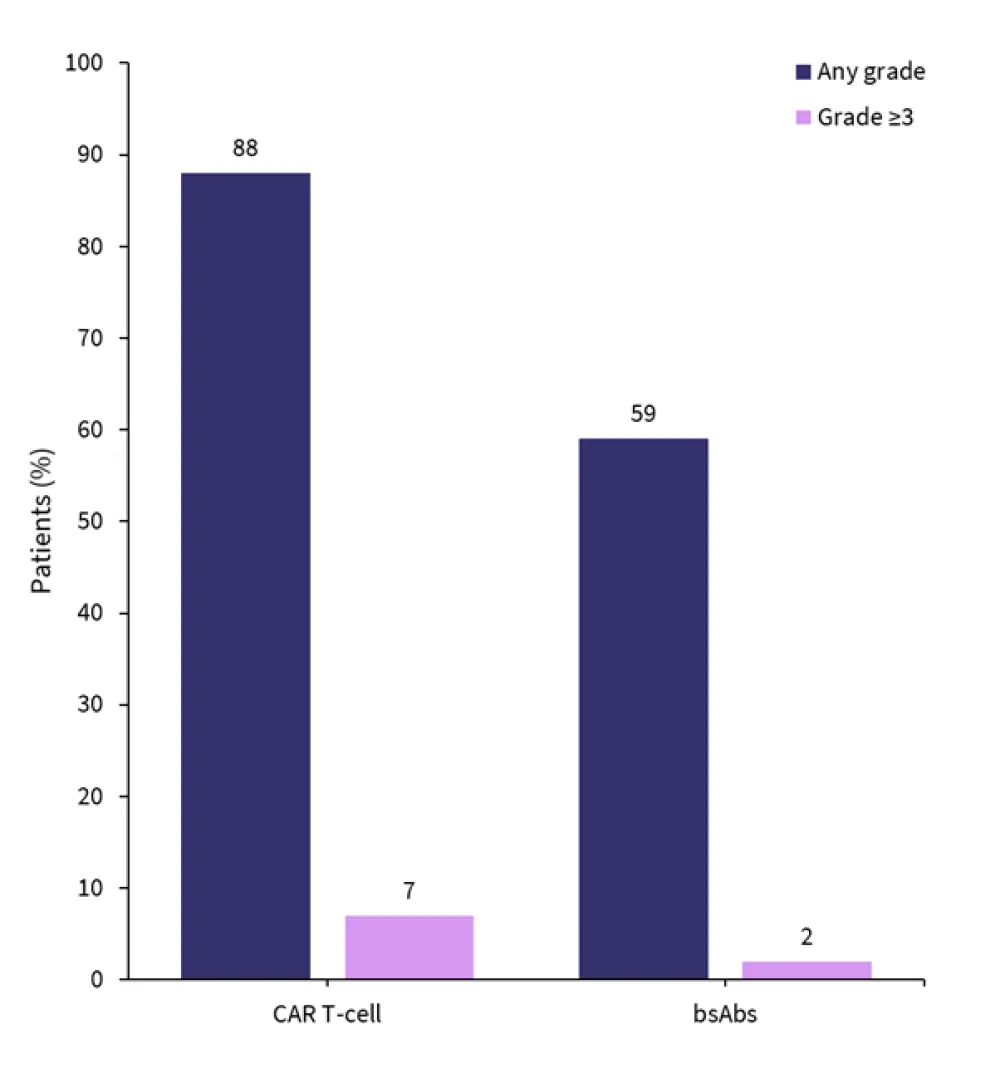
bsAbs, bispecific antibody; CAR, chimeric antigen receptor.
*Data from Soltantabar, et al.1
Table 1. Incidence of CRS by agent and dose level*
|
CRS, cytokine release syndrome; IV, intravenous; SC, subcutaneous. |
|||
|
Drug |
Dose level |
Any grade CRS, % |
Grade ≥3 CRS, % |
|---|---|---|---|
|
Elranatamab |
12/32/76 mg |
56.3 |
0 |
|
4/20/76 mg |
64.4 |
0 |
|
|
44/76 mg; no premed |
100 |
0 |
|
|
44/76 mg premed |
78.3 |
4.3 |
|
|
1,000 μg/kg (76 mg) |
100 |
0 |
|
|
600 μg/kg (44 mg) |
100 |
0 |
|
|
Linvoseltamab |
5/20/50 mg IV |
54.8 |
1.9 |
|
5/20/200 mg IV |
45.3 |
0.8 |
|
|
Alnuctamab |
3/6/10 mg SC |
72 |
0 |
|
3/6/30 mg SC |
44 |
0 |
|
|
3/6/10 mg IV |
71 |
2 |
|
|
ABBV-383 |
40 mg IV |
69 |
0 |
|
60 mg IV |
70 |
2 |
|
|
Key learnings1 |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




