All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Identifying PCL-like MM
Do you know... What is the sensitivity of the transcriptomic classifier developed by Hofste op Bruinink et al. for primary plasma cell leukemia (pPCL)?
Primary plasma cell leukemia (pPCL) is an aggressive clinically high-risk subtype of multiple myeloma (MM) currently characterized by ≥5% circulating tumor cells (CTCs).1,2 The Multiple Myeloma Hub previously reported on the current status and future directions in the diagnosis and treatment of patients with pPCL.
Patients with MM who are symptomatic but have lower CTC levels at diagnosis are classified as having newly diagnosed MM (NDMM) rather than pPCL, but they may experience an equally aggressive disease course.1,2
Hofste op Bruinink et al.1 hypothesized in 2022 that a molecular marker for pPCL could help to identify patients with NDMM with high-risk PCL-like disease in the absence of clinical recognition of pPCL.1 They constructed a transcriptional classifier for PCL-like disease and evaluated its prognostic value in the context of conventional NDMM high-risk markers.1
Further to this, Jelinek et al.2 hypothesized in 2023 that pPCL does not represent a separate clinical entity, but rather a very high risk, poor prognosis MM characterized by elevated CTC levels.2 Here, we summarize key findings from these studies, with a focus on their clinical implications.
Identification of high-risk MM with a PCL-like transcriptomic profile1
Methods
The study by Hofste op Bruinink et al.1 was a retrospective cohort study conducted in the following two phases:
- First, a transcriptomic classifier for PCL-like disease was constructed bioinformatically and validated using data on tumor burden, tumor transcriptomics, and baseline CTC levels in a total of 183 patients from three clinical trials (Figure 1).
- Next, the prognostic value of the transcriptomic classifier was assessed in an independent cohort of 2,139 patients diagnosed with NDMM from seven distinct clinical trials (Figure 1).
Figure 1. Patient selection and classifier construction*
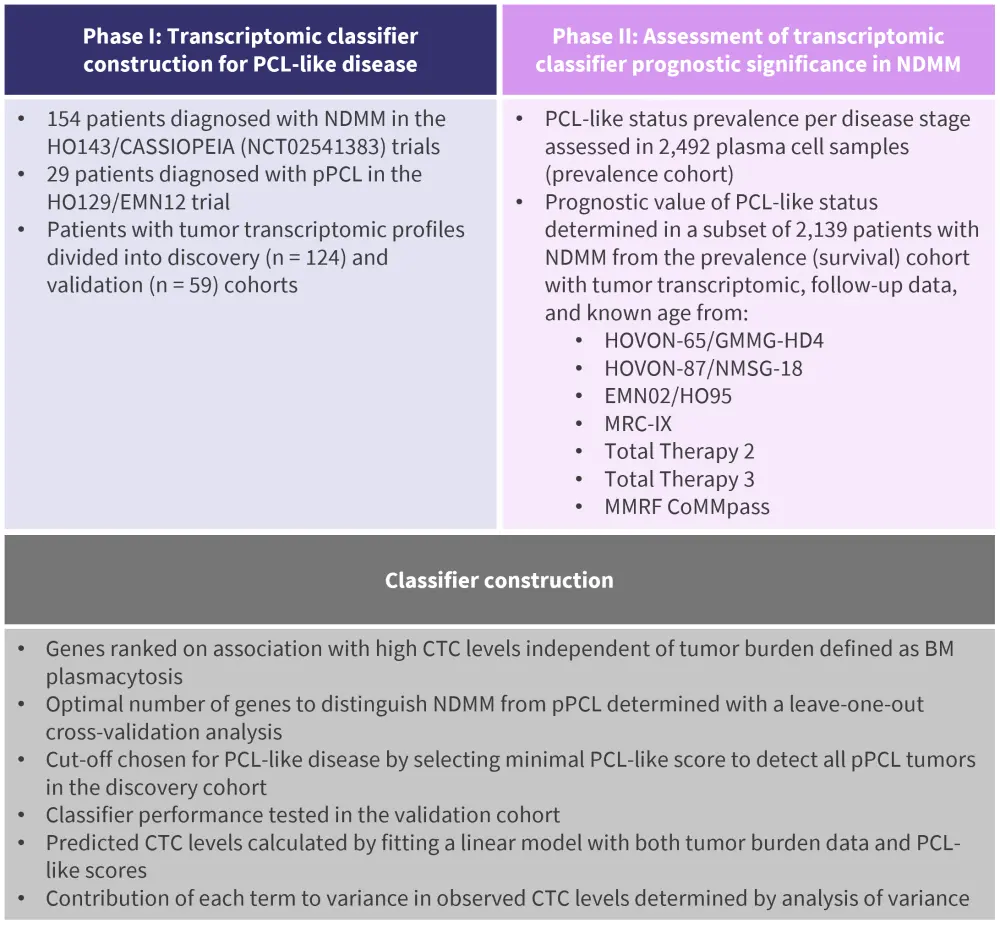
BM, bone marrow; CTC, circulating tumor cell; NDMM; newly diagnosed multiple myeloma; PCL, plasma cell leukemia; pPCL, primary PCL.
*Data from Hofste op Bruinink, et al.1
Results
- High CTC levels were associated with the expression of 1,700 genes in a tumor burden-independent manner.
- 54 of these genes were selected by leave-one-out cross-validation to enable the construction of a transcriptomic classifier representative of PCL-like disease.
- The transcriptomic classifier demonstrated a 93% sensitivity for pPCL identification in the validation cohort and also classified 10% of NDMM tumors as PCL-like.
- PCL-like MM resembled pPCL cytogenetically and transcriptionally, despite presenting with significantly lower tumor burden and CTC levels.
- Multivariate analyses of NDMM data confirmed that PCL-like status demonstrated significant prognostic value regarding both progression-free survival (PFS; hazard ratio, 1.64; 95% confidence interval, 1.30–2.07) and overall survival (OS; hazard ratio, 1.89; 95%, confidence interval, 1.42–2.50) in the context of Revised International Staging System stage, treatment, and age.
Defining PCL–like MM by ≥2% CTCs2
Methods
- To establish a CTC cutoff that would identify patients with ultra-high-risk PCL-like MM, Jelinek et al.2 assessed CTC levels in 395 patients with transplant-ineligible NDMM by multiparameter flow cytometry.
- The cutoff was tested in 185 patients with transplant-eligible MM and validated further in an independent transplant-ineligible cohort of 280 patients treated in the GEM-CLARIDEX trial (NCT02575144).
- The largest published real-world cohort of patients with pPCL was used for survival comparison.
- The current ≥5% threshold for diagnosis of pPCL was challenged.
Results
- Patients with transplant-ineligible NDMM and 2–20% CTCs had significantly shorter PFS (3.1 months vs 15.6 months; p < 0.001) and OS (14.6 months vs 33.6 months; p < 0.023) than patients with <2% CTCs.
- The 2% CTC cutoff was also applicable in transplant-eligible patients with MM and was validated successfully in an independent cohort of patients from the GEM-CLARIDEX trial.
- Patients with 2–20% CTCs experienced very poor outcomes comparable with those observed in patients with pPCL.
- The study revealed a low mean difference between morphologic and flow cytometric CTC evaluation and demonstrated that patients with 2–5% CTCs experience similar outcomes to those with 5–20% CTCs.
Conclusion
Hofste op Bruinink et al.1 demonstrated that pPCL can be identified molecularly as well as clinically. The specific tumor transcriptome allowing pPCL molecular identification was also found in patients with high-risk NDMM despite their lack of clinical leukemia.1 This subgroup of patients with NDMM with a similar tumor transcriptome to pPCL were classified as having PCL-like MM.1 PCL-like status was associated with inferior PFS and OS in NDMM; thus, incorporating this classification into current NDMM risk models may improve prognostic accuracy.1
Jelinek et al.2 revealed that ≥2% CTCs represents a biomarker of hidden pPCL. This lower cutoff threshold identified a small subset of patients with NDMM with ultra-high-risk disease resembling features of pPCL, supporting the assessment of CTCs by flow cytometry during MM diagnostic workup.2 Patients in this subset require more intensive treatments and maintenance regimens, highlighting the clinical importance of their identification.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




