All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Current status and future directions in the diagnosis and treatment of patients with primary PCL
Primary plasma cell leukemia (pPCL) is an aggressive plasma cell disorder and is characterized by a poor prognosis.1 PCL is classified as primary when the leukemic phase presents at diagnosis and secondary when the leukemic transformation occurs in the presence of pre-existing multiple myeloma (MM).1
The diagnostic criteria for PCL were originally defined by >20% circulating plasma cells (CPCs) and an absolute count >2 x 109/L plasma cells in peripheral blood.1 The World Health Organization and the International Myeloma Working Group (IMWG) currently recommend using just one of the two original criterion.2 However, recent studies have suggested that the presence of ≥5% CPCs but not meeting the 20% cutoff has a similar adverse prognostic impact and poor survival as patients with >20% CPCs, warranting a consensus on PCL definition.1 In addition, evidence is limited for the treatment of patients with pPCL, as most are excluded from current MM clinical trials.1 However, of note, the OPTIMUM/MUKnine trial (NCT03188172), summarized by the Multiple Myeloma Hub, investigated the efficacy of daratumumab + cyclophosphamide + lenalidomide, bortezomib + dexamethasone in patients with ultra-high-risk MM and primary PCL.
Fernández de Larrea et al.2 recently published a revised definition proposed by the IMWG for diagnosing pPCL based on peripheral blood plasma cell percentage. In addition, Tuazon et al.1 published a review article on the current status and future directions of pPCL. Here, we summarize the two articles, providing an overview of the clinical manifestation of pPCL, diagnostic evaluation including the proposed new definition, and treatment approaches for patients with pPCL.
Clinical manifestation
Patients with pPCL present with distinct biological and clinical features is shown in Table 1.1,2 The median age at diagnosis is 61 years, which is nearly 10 years younger than the average age of a typical patient with MM.1
Table 1. Clinical and biological features*
|
Del, deletion; HLA-DR, human leukocyte antigen-DR isotype; LDH lactate dehydrogenase. |
|
Features of PCL |
|---|
|
Significant anemia |
|
Thrombocytopenia |
|
Renal insufficiency |
|
Hypercalcemia |
|
Increased tumor burden (increased LDH) and β2-microglobulin |
|
Increased expression of CD20, CD27, CD28, and CD45 |
|
Decreased expression of CD9, CD56, CD117, and HLA-DR |
|
Increased genetic abnormalities such as del(17p), hypodiploidy, and t(11;14) |
|
Reduced bone marrow reserve |
|
Extramedullary involvement |
|
Light chain and non-secretory subtypes |
Diagnostic evaluation
The initial diagnostic evaluation of pPCL is the same as that for MM and is shown in Figure 1.1
Figure 1. Diagnostic evaluation of pPCL*
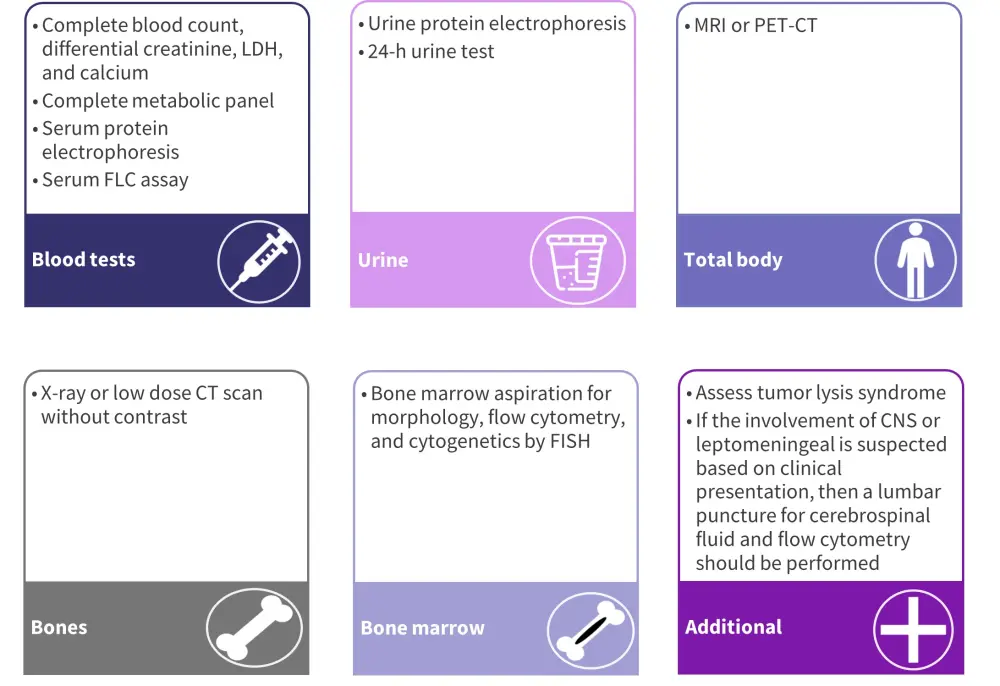
CNS, central nervous system; CT, computed tomography; FISH, fluorescence in situ hybridization; FLC, free light chain; LDH lactate dehydrogenase; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-CT.
*Adapted from Tuazon, et al.1
Proposed definition of primary PCL from the IMWG2
Study design
This was a retrospective analysis of two case control studies (performed in Catalonia, Spain, and Mayo Clinic, US) investigating the outcomes of patients with lower CPC thresholds. The Catalonian study included 100 patients and 382 controls diagnosed with newly diagnosed MM or with reported PCL between 2008 and 2013. The Mayo Clinic study included 176 patients diagnosed with MM between 1971 and 2006 who had CPC at diagnosis and were compared with a historical control of 9,724 patients diagnosed with MM in the same period without CPC.
The primary outcome of the analysis was overall survival (OS) defined from the date of diagnosis to the date of death or last follow-up.
Results
Baseline characteristics
Patients in the Catalonian study were slightly older and had a different sex distribution compared with the Mayo Clinic study (Table 2).
Table 2. Patient characteristics*
|
CPC, circulating plasma cell. |
||
|
Characteristic, % (unless otherwise stated) |
Catalonian study |
Mayo clinic study |
|---|---|---|
|
Median age, years |
69 |
62 |
|
Male/Female |
42/58 |
56/44 |
|
Number of patients with CPC |
100 |
176 |
|
Distribution according to CPC§ |
|
|
|
1–4 |
83 |
31 |
|
5–20 |
12 |
36 |
|
>20 |
5 |
34 |
OS according to CPC
The median follow-up period was longer in the Mayo Clinic study compared with the Catalonian study (6.8 years vs 28 months). The median OS in the different categories based on the percentage of CPCs are shown in Table 3.
Table 3. Overall survival based on the CPC percentage*
|
CPC, circulating plasma cell. |
||
|
Overall survival, months |
Catalonian study |
Mayo clinic study |
|---|---|---|
|
CPC |
|
|
|
0% |
47 |
53 |
|
1–4% |
50 |
17 |
|
5–20% |
6 |
13 |
|
>20% |
14 |
13 |
1–4% CPC
In the Catalonian study, the median OS observed in patients with (1–4%) and without CPCs (50 months vs 47 months, respectively) was similar. In contrast, the Mayo Clinic study showed a major difference in the median OS, with a much lower median OS in patients with 1–4% CPCs compared with those without CPCs (17 months vs 53 months). Interestingly the median OS of patients with 1–4% CPCs was similar to the median OS of patients with >5% CPCs (17 months vs 13 months).
It is worth noting that the follow-up for the Mayo Clinic study was 6.8 years, whereas the Catalan study had a median follow-up of 28 months. Therefore, the median OS for patients with <5% CPCs in the Catalonian group is an estimate.
>5% CPC
In the Catalonian study, OS was significantly shorter in patients with ≥5% CPCs compared with patients with no CPCs at diagnosis or 1–4% CPCs (1.1 years vs 4.4 years; relative risk, 4; p < 0.001) in the Catalonian study. Similarly, the Mayo Clinic study demonstrated shorter survival in patients with >5% CPCs compared with those without or with 1–4% CPCs (1.17 years vs 4.8 years; p < 0.001).
5–19% CPC
Patients with 5–19% CPCs retained the diagnostic significance in the Catalonian study and were treated with novel agents. However, median OS was significantly lower in patients with ≥5% CPCs compared with those with standard and high-risk MM in the Mayo Clinic study (1.4 years vs 7.5 years; p < 0.001). Multivariate analysis showed the presence of 5–20% CPCs was an independent prognostic feature for predicting a shorter OS (relative risk, 4.9), irrespective of age, creatinine, Durie-Salmon system stage, and International Staging System stage.
Consensus recommendations for the proposed definition of pPLC
Based on these findings, the IMWG proposes that the existing definition of pPCL should be revisited and makes recommendations, as shown in Figure 2.
Figure 2. Consensus recommendations for the proposed definition of pPLC*
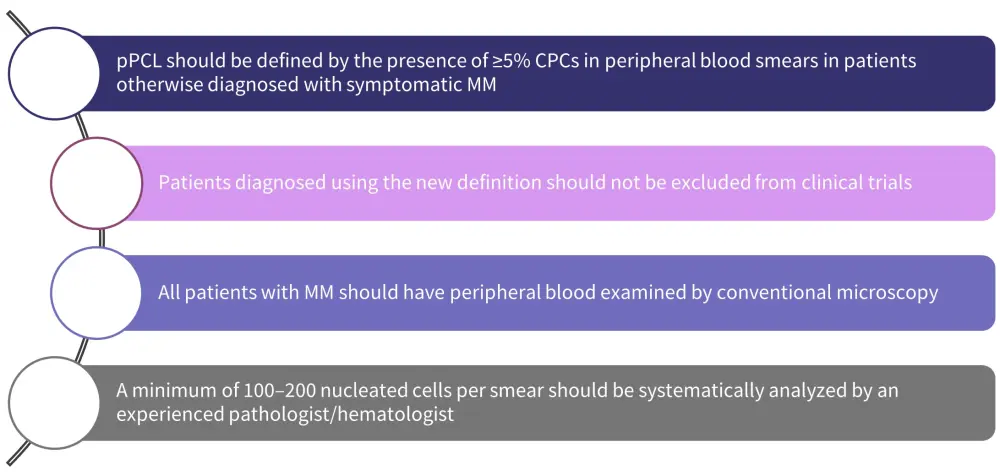
CPC, circulating plasma cell; MM, multiple myeloma; pPCL, primary plasma cell leukemia.
*Adapted from Fernández de Larrea, et al.2
Treatment approaches1
Based on the current evidence, Tuazon et al.1 recommend that treatment for patients with primary PCL should include a combination therapy incorporating a proteasome inhibitor, immunomodulatory drugs (IMiDs), steroids, and/or anthracyclines and alkylators as part of more intensive chemotherapy, followed by consolidative autologous hematopoietic stem cell transplantation (auto-HSCT) in eligible patients, and then maintenance therapy (Figure 3).
Figure 3. Treatment algorithm*
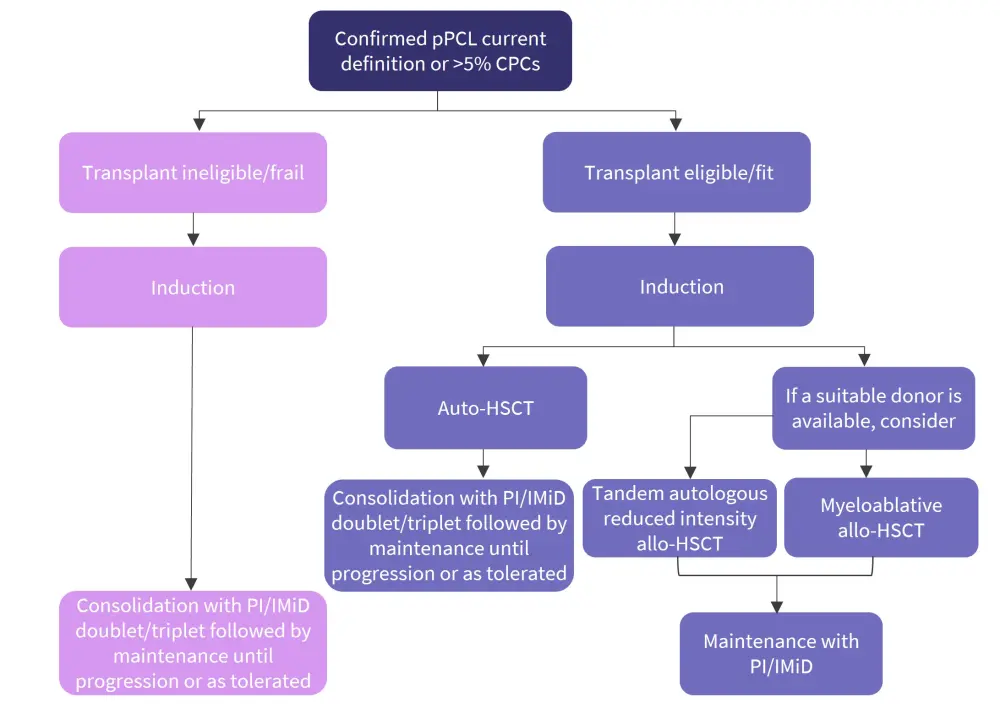
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; auto-HSCT, autologous hematopoietic stem cell transplantation; CPC, circulating plasma cell; IMiD, immunomodulatory drug; PI, proteasome inhibitor; pPCL, primary plasma cell leukemia.
*Adapted from Tuazon, et al.1
Induction
In patients with newly diagnosed pPCL, alternating cycles of bortezomib, dexamethasone + cyclophosphamide, or doxorubicin have shown to be beneficial in a phase II study of 27 patients, in which an overall response rate (ORR) of 69% was achieved.
In addition, IMiDs such as lenalidomide have demonstrated high ORR in patients with pPCL. An ORR of 60% was achieved in a phase II study of patients treated with lenalidomide and dexamethasone +/- high dose-melphalan and ASCT.
Next-generation PIs (carfilazomib, ixazomib), IMiDs (pomalidomide), and CD38-directed monoclonal antibodies (daratumumab) have demonstrated high efficacy in MM and can be speculated to provide similar efficacy in pPCL.
The EMN12/HOVON 129 phase II study of KRd as induction, consolidation, and maintenance in patients with pPCL showed a very good partial response or greater response (n = 14) following four cycles of KRd induction. The addition of newer agents may help to optimize the induction treatment and improve outcomes. Induction therapy should be administered until patients achieve optimal response in those that are eligible for transplant. For patients ineligible for transplant, 8–12 cycles of induction therapy are recommended prior to maintenance therapy initiation.
Intensive chemotherapy regimens may be preferred if there is a pressing need for cytoreduction. These include the following:
- Bortezomib, thalidomide or lenalidomide, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide (VTd/RVd-PACE)
- Cyclophosphamide, vincristine, doxorubicin, dexamethasone, lenalidomide, bortezomib (hyperCVAD-RV)
For frail patients, reduced-intensity regimens, such as RVd + liposomal doxorubicin or RVd + cyclophosphamide, are preferred.
Several new MM therapies are being explored for pPCL and include the following:
- Pomalidomide with ixazomib (NCT02547662)
- Daratumumab combined with bortezomib, dexamethasone, pegylated liposomal doxorubicin, and lenalidomide (NCT03591744)
HSCT
Studies from the European Group for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research have demonstrated improved OS in patients who underwent auto-HSCT. There was a trend towards improved responses in patients that underwent tandem auto-HSCT compared with a single auto-HSCT. However, intensification of treatment with auto-HSCT is still required to improve pPCL outcomes.
Reports on the efficacy of allo-HSCT have been varied. In a report by the Center for International Blood and Marrow Transplant Research (CIBMTR), patients who underwent an allo-HSCT (n = 50) showed a reduced risk of relapse versus auto-HSCT (38% vs 61%), but a poorer 3-year OS (39% vs 64%).3
If allo-HSCT is offered, it should be given early as OS is very poor for patients transplanted after relapse. Treatment-related mortality can also be high in patients with MM undergoing allo-HSCT especially if they are given myeloablative conditioning regimens. On the other hand, patients given reduced-intensity conditioning regimens are at greater risk of relapse. Tandem-auto with myeloablative conditioning/allo-HSCT with reduced-intensity conditioning may be one option for patients to benefit from the cytoreductive properties of one treatment and then rely on the graft versus myeloma effect.
Maintenance therapy
To prevent disease progression, maintenance therapy and/or consolidation post-auto-HSCT should be given within ~60–80 days. Doublet or triplet regimens are recommended, particularly as single agent maintenance, such as lenalidomide, results in relapse rates of ~80% at 2–12 months.
While maintenance therapy after allo-HSCT is controversial, it may be beneficial in the interim before the full graft-versus-myeloma response takes effect. Bortezomib may be preferred in this setting as it may decrease the risk of graft-versus-host disease, as opposed to lenalidomide which may increase the risk.
Assessing response to treatment
No treatment response criteria exist for PCL and those used to assess MM are inadequate for monitoring the extramedullary or leukemic aspects of the disease.
The IMWG have suggested adding peripheral blood PCs and assessment of extramedullary disease to the bone marrow and biomarker criteria used (Table 4).
Table 4. IMWG PCL response criteria*
|
BM, bone marrow; CR, complete response; PB, peripheral blood; PC, plasma cells; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response. |
||||
|
Response |
Serum and urine biomarkers† |
BM PCs |
PB PCs |
Extramedullary disease |
|---|---|---|---|---|
|
sCR |
Negative serum and urine immunofixation; normal serum-free light chain ratio |
<5% by morphology and no malignant PCs by flow cytometry |
Negative by morphology and flow cytometry |
None |
|
CR |
Negative serum and urine immunofixation‡ |
<5% by morphology |
Negative by morphology |
None |
|
VGPR |
≥90% reduction of serum M protein and <100 mg/24 h urinary M protein§ |
<5% by morphology |
Negative by morphology |
None |
|
PR |
≥50% reduction of serum M protein and reduction in 24 h urinary M protein by ≥90% and <200 mg/24 h‖ |
5–25% by morphology |
1–5% PCs by morphology |
≥50% reduction from baseline |
|
SD |
Not meeting the criteria of PR or PD |
|||
|
PD |
>25% increase in the level of serum M protein with an absolute increase ≥5g/L; 25% increase |
>25% increase or absolute increase ≥10% |
>5% absolute increase in PCs by morphology |
Increase in size or number |
|
Relapse from CR |
Reappearance of original M protein in serum and/or urine |
>10% increase |
Detectable at any level |
Any extramedullary disease |
Treating relapse
For the treatment of relapse, patients should be given a combination of agents that they have not previously been exposed to or are not refractory to. In cases where a patient has previously experienced a prolonged response to a particular therapy and relapsed off treatment, re-administering this agent may be an acceptable strategy. Combining novel agents or regimens that include chemotherapy and steroids may be beneficial in this population.
Future directions
As t(11;14) may occur in patients with PCL, treatment with venetoclax may be used. In phase I trials of patients with relapsed/refractory MM, venetoclax had some success in demonstrating efficacy as a single treatment agent.
Immunotherapies such as chimeric antigen receptor T-cell therapy may also have the potential for treatment of PCL, especially B-cell maturation antigen targeting agents. However durable anti-myeloma T-cell responses have yet to be demonstrated.
Other phase II trials for pPCL have looked at combinations with the following:
- NK cells, elotuzumab, lenalidomide, and high-dose melphalan before ASCT (NCT01729091)
- Panobinostat, gemcitabine, busulfan, and melphalan before ASCT (NCT02506959)
Conclusion
The IMWG proposes a new diagnostic definition for pPCL, which being less restrictive would increase the number of patients being diagnosed with pPCL up to three-fold. Patients otherwise classified as symptomatic MM should be enrolled early into clinical trials using the newest immunotherapies and combined strategies to improve survival. However, the limitation of the two studies included by de Larrea et al.2 was that most patients included were not treated with novel agents and/or auto-HSCT. Therefore, prospective studies are needed to understand the molecular characteristics of PCL to gain an improved understanding of the genomic mechanism as a basis to develop optimal stratified treatment approaches. Further research is also needed to establish the role of emerging immunotherapies including chimeric antigen receptor T cells, monoclonal antibodies, bispecific T-cell engagers, antibody–drug conjugates, and small molecule inhibitors such as venetoclax in the treatment of pPCL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

