All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Etiologies and management of cytopenias following CAR T-cell infusion
Do you know... Variable etiologies are associated with cytopenias which can be classified into three types (early, prolonged, and late), based on the timeline from chimeric antigen receptor (CAR) T-cell infusion. Which of the following etiologies is associated with early cytopenia?
Cytopenias are the most common adverse event occurring across a range of hematologic tumors in patients receiving chimeric antigen receptor (CAR) T-cell therapy.1 Cytopenias are associated with increased morbidity and mortality, risk of infections, and bleeding complications. They may also lead to additional disease sequelae, including poor quality of life and increased use of healthcare resources. Various etiologies are associated with cytopenias, some of which are not well understood, which presents challenges and uncertainties around the management.1
During the International Myeloma Society (IMS) 4th Immune Effector Cell Therapies in Multiple Myeloma Workshop, Lin1 presented a session on the real-world considerations for commercial CAR T-cell therapies, including the etiologies and management of cytopenias.1 In the presentation, Lin discusses an article published in Blood by Jain et al.2 on the management of cytopenias post CAR T‑cell infusion. Here, we summarize the potential etiologies and key recommendations for the management of cytopenias.
Etiologies and management of cytopenias1,2
Cytopenia may be caused by several underlying pathophysiological mechanisms and can be classified into three types by considering the timeline from CAR T-cell infusion. Figure 1 shows the potential underlying etiologies and recommended management strategies proposed by Jain et al.2
Figure 1. Etiologies and recommended management strategies of cytopenia*
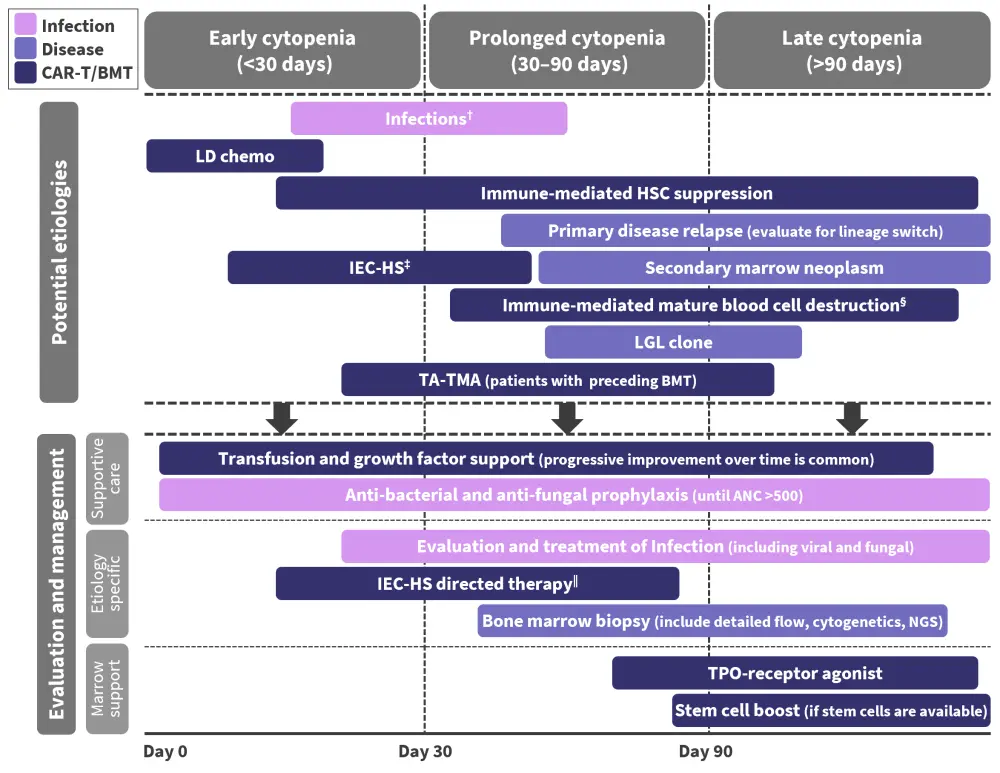
ANC, absolute neutrophil count; CAR, chimeric antigen receptor; G-CSF, granulocyte colony stimulating factor; IEC-HS, hemophagocytic lymphohistiocytosis-like hyperinflammatory syndrome associated with immune effector cells; LD, lymphodepletion; LGL, larger granular lymphocytosis; NGS, next-generation sequencing; PJP; Pneumocystis jirovecii pneumonia; TA-TMA, transplant-associated thrombotic microangiopathy; TPO, thrombopoietin.
*Adapted from Jain, et al.2
†Anti-viral prophylaxis initiated early post CAR T-cell infusion and continued for 6–12 months; anti-PJP prophylaxis initiated at Day ~28 until CD4+ cell count >200/mm3; if ANIC <500/mm3 by day 7–10, consider administering G-CSF.
‡Rapidly rising ferritin, fever, organ dysfunction.
§Remain vigilant.
‖Consider anakinra, steroids, ruxolitinib.
Early cytopenia (<30 days from infusion)
Etiology
Most cases of early cytopenia occur as a result of CAR T-cell expansion in vivo by lymphodepletion (LD) chemotherapy, inducing a lymphopenic environment. This increases homeostatic cytokines and decreases the suppressive cells. Although LD is not myeloablative, and recovery of blood count is expected following chemotherapy, cytopenias may continue or persist beyond those associated with LD alone. Cytopenia can be biphasic, with patients experiencing a second occurrence of reduced absolute neutrophil count (ANC) between Week 3 and Month 1, termed as intermittent neutrophil recovery. This is also observed in many patients with prolonged cytopenia after CAR T-cell infusion. A small proportion of patients may present with suboptimal count recovery post-nadir that is less responsive to growth factors. In these patients, late cytopenia is common and a full recovery may not occur until months later.
Recommended treatment for early cytopenia
- Supportive treatment with platelet and packed red blood cell and platelet transfusions
- Comprehensive evaluation and management of infections, including viral and fungal infections
- Bacterial and fungal prophylaxis on Day 0, or at the time of first neutropenia, until ANC recovery
- Initiation of early antiviral prophylaxis after CAR T-cell infusion, continued for 6–12 months
- Anti-pneumocystis jirovecii pneumonia (PJP) prophylaxis at Day 30, encouraging discontinuation when CD4+ count is >200/mm3
- Consider pentamidine or atovaquone for PJP prophylaxis
- If ANC is <500/mm3 by Day 7–10, consider administration of granulocyte colony stimulating factor (G-CSF)
- G-CSF should be avoided in patients with ongoing cytokine release syndrome (CRS) and immune cell-associated neurotoxicity syndrome
Prolonged cytopenia (30–90 days from infusion)
Etiology
Prolonged and late (>90 days) cytopenia after CAR T‑cell infusion may be caused by immune-driven suppression of hematopoietic stem cells and marrow microenvironment changes. Prolonged cytopenia is associated with preceding cytopenia, either disease- or chemotherapy-related, at the time of CAR T‑cell infusion. Marrow reserves at the time of CAR T‑cell infusion may also increase the probability of developing prolonged cytopenia. Patients undergoing allogeneic bone marrow transplantation (BMT) with decreased marrow are susceptible to prolonged cytopenia. Residual or recurrent disease in the marrow, high marrow disease, and the co-stimulatory domain of the CAR may lead to prolonged cytopenia.
Infections and immune effector cell-associated hemophagocytic lymphistiocytosis-like syndrome (IEC-HS) should be considered in both early and prolonged cytopenia. IEC-HS should also be monitored in patients with previously resolved CRS followed by a rapid increase in ferritin (>10,000 mg/mL), recurrence of fever, and new onset of severe cytopenia.
The following biomarkers that are consistent across both early and prolonged cytopenia should be considered:
- Higher levels of serum ferritin levels and C-reactive protein correlate with lower probability of complete white blood cell count, hemoglobin, platelet, and neutrophil count recovery.
- Interleukin-6 and other cytokines detect cytokine-mediated dysfunction.
- CAR T-HEMATOTOX score, which uses blood and inflammatory markers, can be helpful in predicting patients with multiple myeloma with prolonged and high risk of cytopenia after CAR T-cell infusion, as highlighted by Lin.1
Recommended treatment for prolonged cytopenia
The most likely trajectory for patients with prolonged or late cytopenia in the absence of additional pathophysiological causes is gradual recovery. These patients should be monitored for active primary disease in the marrow or secondary myeloid malignancies. With regards to patients with prolonged cytopenia, the following should be considered:
- Prolonged neutropenia or active infection may be treated with G-CSF.
- Transient responses are common suggesting gradual improvement and decreased dependence on G-CSF.
- However, a complete lack of response to G-CSF may prompt BM biopsy.
- BM aspiration should be considered when there is a high likelihood of primary disease progression/relapse, marrow fibrosis or lineage dysplasia before CAR T-cell infusion or presence of IEC-HS.
- In case IEC-HS is suspected, anakinra and high-dose steroids may be considered.
- Refractory IEC-HS may respond to ruxolitinib or cyclophosphamide or etoposide but is based on anecdotal evidence.
- Thrombopoietin-receptor agonists (eltrombopag 75 mg daily with consideration for titration to 150 mg or romiplostim 10 mg/kg/week) may be considered in patients without clear etiology and with high risk of bleeding and hemorrhage.
- Thrombopoietin-receptor agonists should be stopped once platelets recover or if no response within 4–6 weeks after initiation.
- The role of thrombopoietin-receptor agonists in cytopenias needs to be further investigated.
Late cytopenia (>90 days from infusion)
Etiology
Late cytopenias are associated with a hypoplastic marrow without evidence of fibrosis, dysplasia, or clonal hematopoiesis of indeterminate potential (CHIP). Persistent CAR activation may cause immune-mediated mature blood cell destruction, leading to late cytopenia accompanied by severe trilineage aplasia.
Recommended treatment for late cytopenia
- Evaluation and intervention as recommended for prolonged cytopenia.
- Bacterial and fungal infection prophylaxis (fluconazole or micafungin) in patients with severe neutropenia.
- Immunosuppressive agents may be considered in case of immune-mediated dysfunction and cytopenia complicated by large granular lymphocytosis.
- Autologous hematopoietic stem cell transplantation should be considered in severe transfusion dependent late cytopenia and in the absence of secondary malignancy or recurrent primary disease.
- Allogeneic BMT should be considered in patients with aplastic anemia with little evidence of hematopoiesis.
- Monitoring of secondary leukemia including T-cell leukemia arising from viral vector-based gene integration or clonal marrow pathology is recommended.
Paucity of data in the management of prolonged or late transfusion-dependent multi-lineage cytopenias creates a challenge in their management. Although hematopoietic stem cell boost is recommended, this strategy is only feasible if stem cells from previous autologous or an allogeneic BMT have been preserved. However, as stem cell products may be contaminated with primary malignant cells, caution should be taken while considering this option.
Specific patient populations2
Pediatric-specific considerations for prolonged cytopenia
- In the pediatric population, prolonged cytopenia may occur due to lineage switch by certain cytogenetic drivers of B-cell acute lymphoblastic leukemia (B-ALL) clones following CAR T‑cell infusion.
- Marrow morphology, including dysplastic features and fibrosis, along with broad cytogenetic, immunophenotype, and molecular screening, including next-generation sequencing, should be considered in pediatric patients with ALL.
- Sustained immune dysregulation leading to hematopoietic suppression or to immune-mediated mature blood cell destruction is more frequent in pediatric cases.
- Pediatric patients are more likely to undergo allogeneic BMT; therefore, these patients may be considered candidates for BMT post-CAR T-cell therapy to consolidate B-ALL remission or treat cytopenia in severe cases. Prolonged use of growth factors should be avoided in this population as this may worsen CRS.
- Allogeneic BMT with best available donor should be considered in BMT-naïve pediatric patients with transfusion dependence or severe neutropenia requiring G-CSF >90 days after CAR T-cell infusion.
Cytopenia in patients who have undergone previous BMT
- Donor graft may be significantly damaged in pediatric patients with relapsed B-ALL following allogeneic BMT.
- Prolonged graft dysfunction or graft failure may result from LD chemotherapy or interferon-gamma-driven hematopoietic suppression during CRS in patients receiving CAR T-cell infusion post-BMT relapse.
- Immune suppression from LD and CRS may reactivate several viruses. Therefore, use of select anti-viral agents should be considered in the management of pre- or post-CAR T-cell infusion.
- In patients with prolonged, transfusion-dependent anemia and thrombocytopenia with neutrophil recovery, transplant-associated thrombotic microangiopathy (TA-TMA) may be a specific consideration.
- Such patients should undergo formal assessment for TA-TMA, including hypertension and proteinuria assessments.
- For patients with late cytopenias post-CAR T-cell infusion and BMT, strong consideration should be given to stem cell boost, provided the original donor or leftover T-cell depleted CD34+ cells are available.
- Second allogeneic BMT instead of stem cell boost should be considered in patients with adequate organ function post BMT and CAR T-cell infusion.
Conclusion
Cytopenias vary in etiology and non-CAR T-cell infusion causes should be considered when assessing patients. Cytopenias may be associated with severity of inflammatory toxicities, pre-existing cytopenias, previous BMT, and marrow disease. However, further investigations into the underlying mechanism of cytopenias will inform future treatment strategies. Currently, cytopenia should be managed based on its severity and timing post CAR T-cell infusion.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




