All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Elranatamab for RRMM: Updates from the MagnetisMM trials
On August 14, 2023, the U.S. Food and Drug Administration (FDA) granted accelerated approval to elranatamab for the treatment of relapsed/refractory multiple myeloma (RRMM) in patients who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody. This approval was based on data from the MagnetisMM-3 clinical trial which is summarized here alongside the alternative MagnetisMM trials.4
Elranatamab is an investigational B-cell maturation antigen (BCMA) CD3 bispecific antibody currently under investigation in relapsed/refractory multiple myeloma (RRMM) as part of the MagnetisMM trials.1 The latest results from the MagnetisMM trials were presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition,1 the European Hematology Association (EHA) 2023 Hybrid Congress,2 and the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting.3
Here, we summarize key safety and efficacy data from the presentations of elranatamab in RRMM and in BCMA-naïve and -exposed subgroups.
MagnetisMM
The interventions and designs of the MagnetisMM trials included in this review are outlined in Table 1.
Table 1. Study design overview*
|
CRS, cytokine release syndrome; DLT, dose-limiting toxicity; DOR, duration of response; IMiD, immunomodulatory drug; IMWG, International Myeloma Working Group; mAb, monoclonal antibody; ORR, overall response rate; PI, proteasome inhibitor; RRMM, relapsed/refractory multiple myeloma; SQ, subcutaneously. |
|||
|
Trial |
Intervention |
Design |
Endpoint |
|---|---|---|---|
|
Elranatamab as a monotherapy and in combination with IMiDs for RRMM |
Phase I (N = 55) |
DLT, ORR, and DOR |
|
|
MagnetisMM-2 |
Elranatamab as a monotherapy in RRMM |
Phase I (N = 4) |
DLTs at 28 days |
|
MagnetisMM-3 |
Elranatamab as a monotherapy in RRMM refractory to ≥1 PI, IMiD, and anti-CD38 mAb |
Phase II (N = 187) |
ORR every 4 weeks up to 2 years |
|
MagnetisMM-9 |
Elranatamab as a monotherapy or in combination with dexamethasone in RRMM |
Phase I/II (N = 76) |
Incidence of CRS at Day 28 and ORR per IMWG criteria up to 2 years |
Elranatamab in RRMM (MM-1)1
The latest data from MagnestisMM-1 includes 55 patients with RRMM, dosed at 250–1,000 µg/kg either weekly or fortnightly with subcutaneous elranatamab.
At a median follow-up of 12 months, 64% of patients achieved an overall response (ORR), with 10% achieving a complete response (CR), 7.3% a partial response, and 18.2% a very good partial response.
Secondary endpoint data:
- Median duration of response in responders (n = 35): 17.1 months
- Median progression-free survival: 11.8 months
- Measurable residual disease (MRD) negativity at any point: 100%
- MRD negativity at >6 months: 62%
Elranatamab in BCMA-naïve RRMM (MM-3)2
These data include the updated response and safety data at a 15-month follow up of the 123 patients who were BCMA-naïve from the MagnetisMM-3 trial. In the total cohort, an ORR of 61% was recorded, with 34.9% at CR or stringent CR. This cohort was divided into subgroups including by number of previous therapies and presence of extramedullary disease. Responses in these patient subgroups are shown in Figure 1.
Figure 1. Response rates in BCMA-naïve patients*
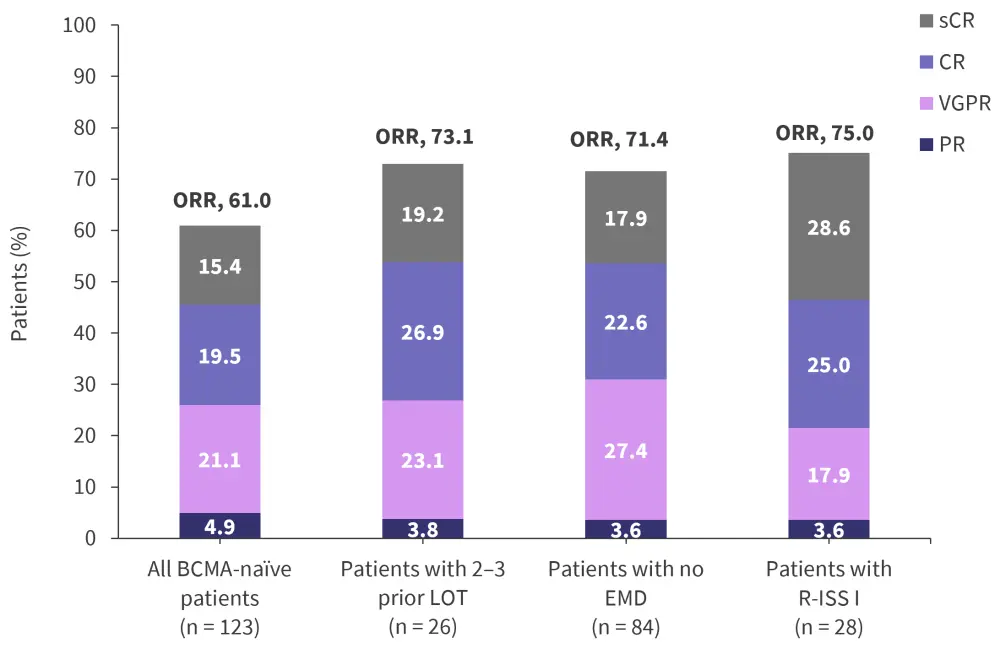
BCMA, B-cell maturation antigen; CR, complete response; EMD, extramedullary disease; LOT, line of therapy; ORR, overall response rate; PR, partial response; R-ISS, revised International Staging System; sCR, stringent complete response; VGPR, very good partial response.
*Data from Mohty.2
Secondary endpoint data:
- MRD negativity at any point in patients who achieved CR/stringent CR (n = 29): 89.7%
- Median time to response in responders (n = 75): 1.2 months
Elranatamab in BCMA-exposed RRMM (MM-1, -2, -3, and -9)3
In a pooled analysis from the MagnetisMM-1, -2, -3, and -9 trials of patients who were exposed to ≥1 proteasome inhibitor, immunomodulatory drug, anti-CD38 antibody, and BCMA-directed therapy; 87 patients were evaluated for their response to elranatamab based on previous BCMA exposure. In the total population of exposed patients, an ORR of 46% was recorded, with a higher ORR of 52.8% in patients previously treated with CAR T-cell therapy (Figure 2).
Figure 2. Response rates in BCMA-exposed patients*
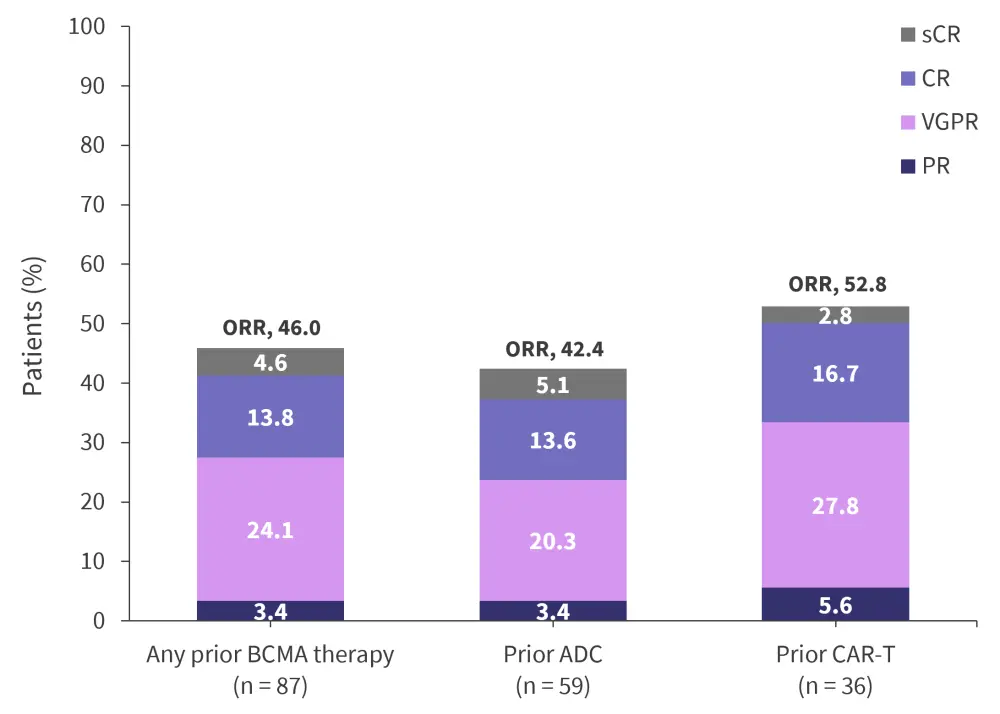
ADC, antibody–drug conjugate; BCMA, B-cell maturation antigen; CAR-T, chimeric antigen receptor T cell therapy; CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Data from Nooka.3
Median progression-free survival, overall survival (OS), and duration of response data are presented in Figure 3, with the prior antibody-drug conjugate and prior CAR T-cell therapy groups provided for comparison.
Figure 3. Secondary endpoint data in BCMA-exposed patients*
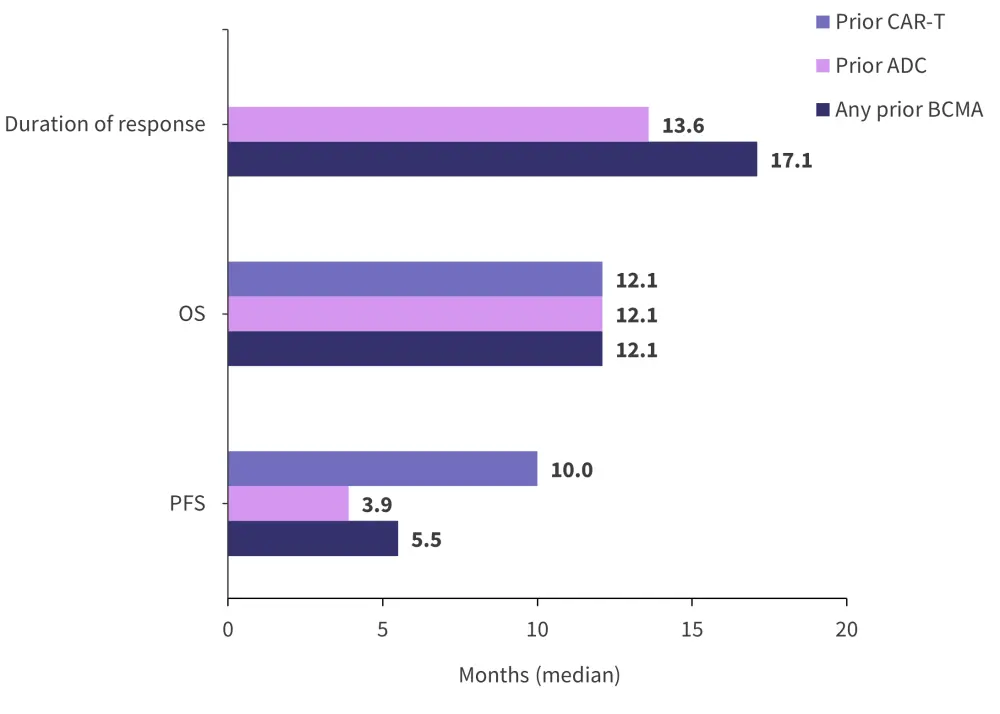
ADC, antibody–drug conjugate; BCMA, B-cell maturation antigen; CAR-T, chimeric antigen receptor T-cell therapy; OS, overall survival; PFS, progression-free survival.
*Data from Nooka.3
Safety of elranatamab1-3
Safety data were deemed manageable by the researchers in all patient subgroups evaluated. Cytokine release syndrome (CRS) was the most common adverse event recorded across all MagnetisMM trials, with 87.3% of patients experiencing CRS in the non-BCMA differentiated population in MagnetisMM-1. Grade 3/4 CRS was exclusively observed in the BCMA-exposed population at 2.3%. Excluding CRS, the most frequently occurring adverse events were hematologic in all populations. Safety data are shown in Table 2.
Table 2. Adverse events*
|
BCMA, B-cell maturation antigen; CRS, cytokine release syndrome. |
||||||
|
Adverse event, % |
Elranatamab in patients regardless of BCMA exposure |
Elranatamab in BCMA-naïve patients |
Elranatamab in BCMA-exposed patients |
|||
|---|---|---|---|---|---|---|
|
Any grade |
≥Grade 3 |
Any grade |
≥Grade 3 |
Any grade |
≥Grade 3 |
|
|
Hematologic |
|
|
|
|
|
|
|
Neutropenia |
74.5 |
70.9 |
48.8 |
48.8 |
44.8 |
41.4 |
|
Anemia |
67.3 |
50.9 |
48.8 |
37.4 |
58.6 |
46.0 |
|
Lymphopenia |
52.7 |
50.9 |
26.8 |
25.2 |
32.2 |
29.9 |
|
Thrombocytopenia |
50.9 |
29.1 |
30.9 |
23.6 |
32.2 |
29.9 |
|
Leukopenia |
— |
— |
— |
— |
21.8 |
12.6 |
|
Non-hematologic |
|
|
|
|
|
|
|
CRS |
87.3 |
0.0 |
57.7 |
0.0 |
65.5 |
2.3 |
|
Diarrhea |
40.0 |
3.6 |
42.3 |
1.6 |
35.6 |
0.0 |
|
Fatigue |
41.8 |
7.3 |
36.6 |
3.3 |
— |
— |
|
Decreased appetite |
34.5 |
1.8 |
33.3 |
0.8 |
23.0 |
1.1 |
|
Injection site reaction |
56.4 |
0.0 |
26.8 |
0.0 |
20.7 |
0.0 |
Conclusion
The latest follow-up data from the MagnetisMM trials in combination support the further evaluation of elranatamab in patients with and without previous BCMA exposure. The findings also highlight patient subgroups that may achieve better responses, such as refractory (2–3 prior lines of therapy) patients in the naïve population and CAR T-cell therapy-treated patients in the exposed population. The differing study designs and participants make drawing direct comparisons between the MagnetisMM trials challenging, and comparisons should therefore be limited to equivalent subgroups within each trial.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




