All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Findings from the MagnetisMM-1 trial of elranatamab in patients with RRMM: An update from ASH 2021
Update/disclaimer: This article has been modified from that published on July 2, 2021, to include updated safety and efficacy data, which were presented by Michael Sebag at the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition.1
Elranatamab (PF-06863135) is a bispecific antibody (IgG2a) targeting the B-cell maturation antigen (BCMA) on multiple myeloma (MM) cells and CD3 on T cells, bridging them together to activate an immune response. The binding affinity of elranatamab to BCMA and CD3 has been optimized to potentially prompt more potent T cell-mediated anti-myeloma activity. Subcutaneous (SC) administration of elranatamab is intended to allow higher doses than intravenous administration without increasing the incidence of adverse events (AEs).
The Multiple Myeloma Hub has previously reported about elranatamab being granted fast track designation by the U.S. Food and Drug Administration (FDA) to aid the rapid development and review, including initial data from the phase I MagnetisMM-1 trial presented during the 62nd ASH Annual Meeting and Exposition. Further results from the MagnetisMM-1 trial were also presented during the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting by Nizar Bahlis2 and the 26th Congress of the European Hematology Association (EHA2021) by Caitlin Costello3. Recently, during the 63rd ASH Annual Meeting and Exposition, Michael Sebag3 presented updated data on the overall response rates (ORRs) and safety of elranatamab. This article provides a summary of the presentation.
Study design
MagnetisMM-1 (NCT03269136) is a phase I, open-label, multicenter, dose-escalation trial to assess the safety and efficacy of elranatamab in adult patients with advanced relapsed or refractory MM (RRMM), who had received prior treatment with immunomodulatory drugs, protease inhibitors, and anti-CD38 therapy.
Patients with measurable disease according to the International Myeloma Working Group (IMWG) criteria, an absolute neutrophil count of ≥1.0 × 109/L, platelets ≥25 × 109/L, and hemoglobin ≥8 g/dL were eligible. Of note, prior BCMA-directed therapy was allowed.
The study was split into three cohorts (Figure 1):
- Dose escalation: patients received 80–1,000 µg/kg elranatamab weekly.
- Priming: patients received a single priming dose of 600 µg/kg elranatamab, followed 1 week later by a full dose of 1,000 µg/kg elranatamab weekly or every 2 weeks.
- Expansion: patients received a fixed, non-weight-based dose of 44 mg elranatamab, followed 1 week later by a full dose of 76 mg elranatamab weekly. Premedication with dexamethasone, diphenhydramine, and acetaminophen was given with priming and full first dose.
The primary endpoints were ORRs (assessed by IMWG criteria), treatment-emergent AEs (assessed by the Common Terminology Criteria for Adverse Events [CTCAE] v4.03), cytokine release syndrome (CRS) incidence (graded by the American Society for Transplantation and Cellular therapy [ASTCT] criteria), dose-limiting toxicity (measured at the end of Cycle 1), and recommended phase II dose (RP2D).
Secondary endpoints included pharmacokinetics and pharmacodynamics.
Figure 1. Dosing schema*
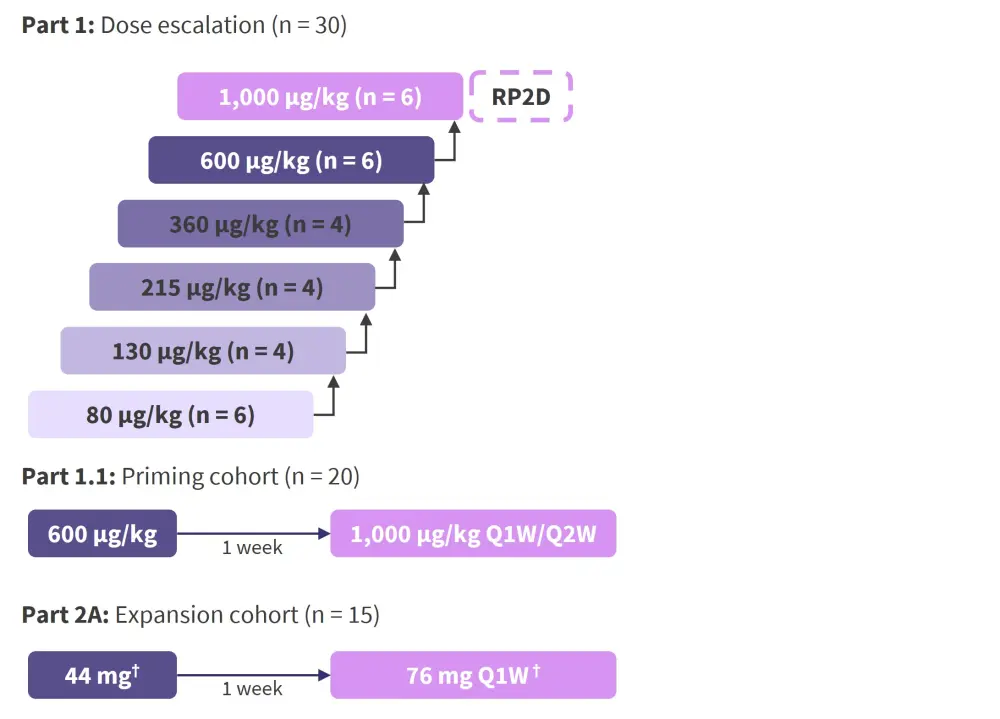
Q1W, once weekly; Q2W, once every 2 weeks; RP2D, recommended phase II dose.
*Adapted from Sebag.1
†Premedication with dexamethasone, diphenhydramine, and acetaminophen was given with priming and full first dose.
Baseline characteristics
At data cutoff (July 26, 2021), 55 heavily pretreated patients treated with a median of six prior therapies (range, 2−15) had received elranatamab at efficacious dose levels (≥215 µg/kg). In total, 49.1% of all patients were ≥65 years of age, and 27.3% of patients had high-risk cytogenetic features, including t[4;14], t[14;16], and del[17p] (Table 1).
Table 1. Baseline characteristics*
|
ADC, antibody–drug conjugate; BCMA, B-cell maturation antigen; CAR-T, chimeric antigen receptor T cell; IMiDs, immunomodulatory drugs; PI, protease inhibitor; R-ISS, Revised International Staging System. |
|
|
Characteristic |
n = 55 |
|---|---|
|
Female, % |
47.3 |
|
Median age (range), years |
64 (42–80) |
|
≥65 years, % |
49.1 |
|
R-ISS stage at initial diagnosis, % |
|
|
I |
25.5 |
|
II |
36.4 |
|
III |
18.2 |
|
Not reported |
20.0 |
|
Cytogenic risk, % |
|
|
High |
27.3 |
|
Median prior anti-myeloma therapies (range) |
6 (2–15) |
|
Prior therapies, % |
|
|
PIs |
100 |
|
Bortezomib |
92.7 |
|
Carfilzomib |
85.5 |
|
Ixazomib |
27.3 |
|
IMiDs |
100 |
|
Lenalidomide |
98.2 |
|
Pomalidomide |
94.5 |
|
Thalidomide |
16.4 |
|
Anti-CD38 therapy |
98.2 |
|
Daratumumab |
94.5 |
|
Isatuximab |
7.3 |
|
Other |
1.8 |
|
BCMA-targeted therapy |
21.8 |
|
Anti-BCMA ADC |
12.7 |
|
CAR-T |
16.4 |
|
Triple-class exposed, % |
98.2 |
|
Triple-class refractory disease, % |
90.9 |
Results
Efficacy
Part 1: Dose escalation cohort
- Responses were observed starting at a dose of 215 µg/kg, and the median follow-up was 12.5 months.
- ORR was 83% at 1,000 µg/kg (RP2D) and 70% in the total efficacious group (Figure 2).
- In 75% of patients (3/4) with prior BCMA-directed therapy, very good partial response (VGPR) was attained in two patients and stringent complete response was achieved in one patient.
- Of the four patients assessed, 100% were minimal residual disease (MRD)-negative at 10−5 by next-generation sequencing.
Figure 2. Investigator-assessed IMWG responses of the dose escalation cohort*
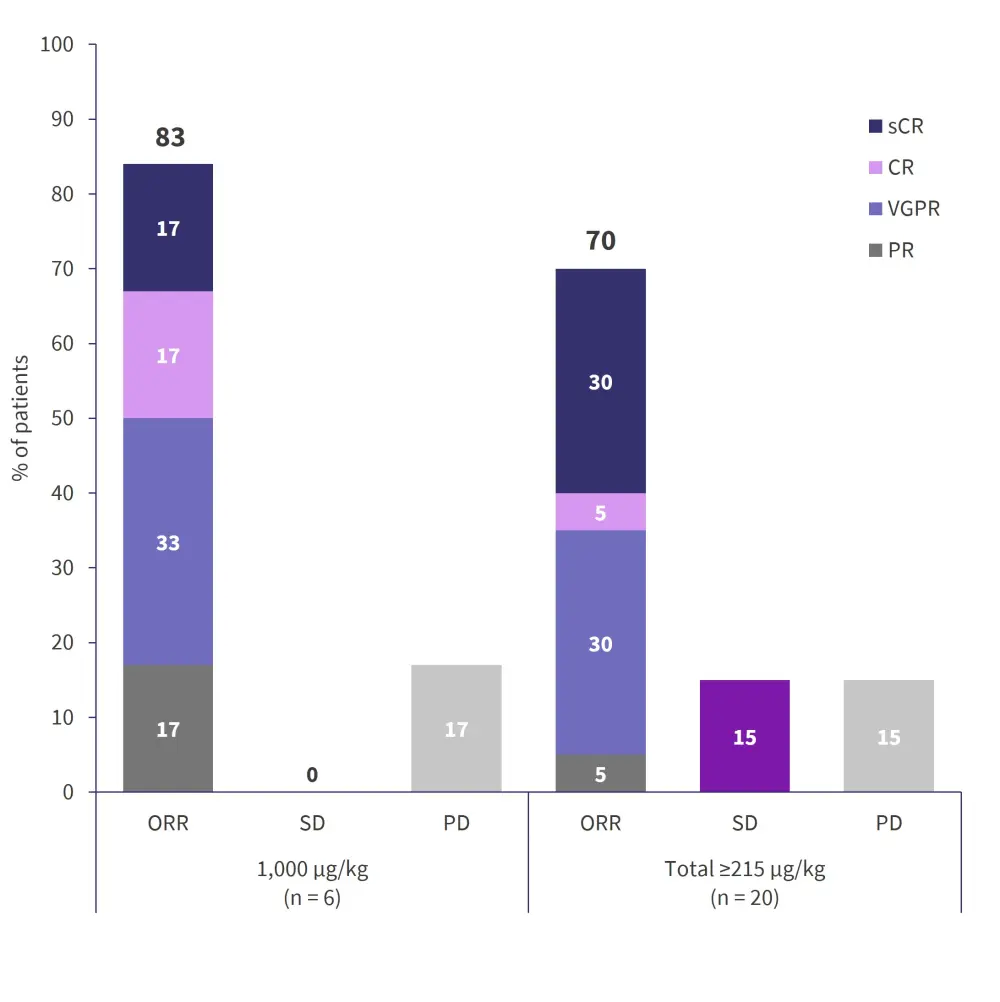
CR, complete response; IMWG, International Myeloma Working Group; ORR, overall response rate; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
*Data from Sebag.1
Part 1.1: Priming cohort
With a median follow-up of 7.5 months, the ORR was 57% for those dosed once a week and 61% for the twice weekly dosing group (Figure 3).
- One patient was not evaluable in each group.
- Of the patients that received prior BCMA-directed therapy, 100% (2/2) in the once a week cohort achieved a response (one achieved a PR and the other a VGPR), and 50% (2/4) in the once every 2 weeks cohort achieved a response (one achieved a complete response and one a VGPR).
- Of the four patients assessed for MRD, all remained MRD negative.
Figure 3. Investigator-assessed IMWG responses of the priming cohort (n = 20)*
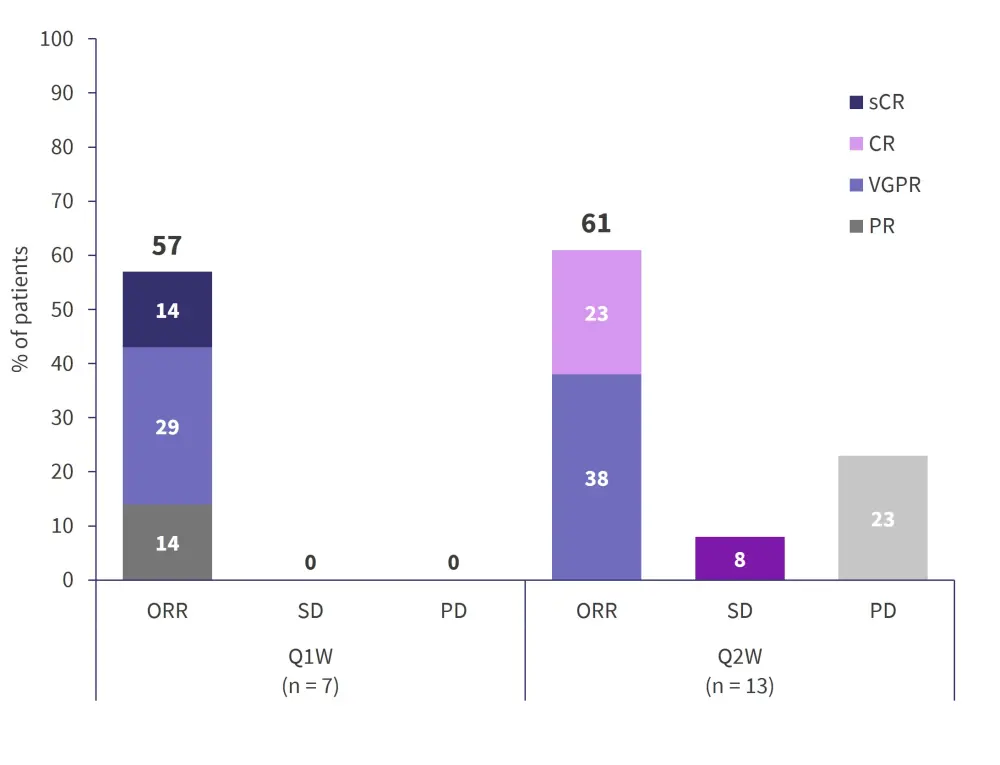
CR, complete response; IMWG, International Myeloma Working Group; ORR, overall response rate; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
*Data from Sebag.1
ORR at the RP2D of 1,000 mg/kg was 69% (9/13 patients: five from the expansion cohort and four from the priming cohort).
Safety
- Treatment-emergent AEs observed in ≥33% of patients are listed in Table 2. Neutropenia (70.9%) was the most common hematologic AE, followed by anemia (65.5%) and lymphopenia (50.9%).
- One dose-limiting toxicity of thrombocytopenia (Grade 4) was reported in the once every 2 weeks priming cohort.
- The most common nonhematologic AE was CRS (87.3%); however, these were limited to Grade 1 or 2 only.
Table 2. Treatment-emergent AEs*
|
AEs, adverse events; CRS, cytokine release syndrome. |
|||||
|
AEs, % |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Total |
|---|---|---|---|---|---|
|
Hematologic |
|
|
|
|
|
|
Lymphopenia |
0 |
0 |
5.5 |
45.5 |
50.9 |
|
Anemia |
3.6 |
14.5 |
45.5 |
1.8 |
65.5 |
|
Neutropenia |
0 |
3.6 |
23.6 |
43.6 |
70.9 |
|
Thrombocytopenia |
10.9 |
10.9 |
9.1 |
18.2 |
49.1 |
|
Leukopenia |
3.6 |
7.3 |
16.4 |
7.3 |
34.5 |
|
Nonhematologic |
|
|
|
|
|
|
CRS |
50.9 |
36.4 |
0 |
0 |
87.3 |
|
Injection site reaction |
49.1 |
7.3 |
0 |
0 |
56.4 |
|
Fatigue |
10.9 |
21.8 |
5.5 |
0 |
38.2 |
|
Diarrhea |
20.0 |
12.7 |
3.6 |
0 |
36.4 |
|
Hypophosphatemia |
0 |
10.9 |
23.6 |
1.8 |
36.4 |
|
Decreased appetite |
21.8 |
10.9 |
1.8 |
0 |
34.5 |
|
Dry skin |
30.9 |
3.6 |
0 |
0 |
33.5 |
The effect of priming and premedication on CRS
- The effect of priming (one step) and premedication in the expansion cohort reduced the overall incidence of CRS by a third (Table 3).
- The median duration of CRS was also reduced by a day (Table 3).
- Priming and premedication also reduced and/or delayed acute cytokine production (including that of interferon γ, tumor necrosis factor-α, interleukin 2, and interleukin 6).
Table 3. Effect of priming and premedication on CRS*
|
CRS, cytokine release syndrome. |
|||
|
|
Dose escalation† |
Priming† |
Expansion† |
|---|---|---|---|
|
Priming/premedication |
No/No |
Yes/No |
Yes/Yes |
|
Incidence of CRS, % |
100 |
100 |
66.7 |
|
Grade 1 |
66.7 |
50 |
33.3 |
|
Grade 2 |
33.3 |
50 |
33.3 |
|
Median duration (range), days |
4 (1–10) |
3 (2–7) |
3 (1–4) |
Pharmacokinetics and pharmacodynamics
- Elranatamab exposure increased in an approximately dose-proportionate manner. A prolonged absorption phase with Tmax of 3−7 days was observed after a single dose administrated subcutaneously.
- Elranatamab 1,000 µg/kg once every 2 weeks achieved exposure in the range associated with anti-myeloma efficacy. This supports the transition of responding patients to once every 2 weeks dosing after 6 months of therapy.
Enrollment is paused temporarily on elranatamab trials due to peripheral neuropathy2,4
Part 2 from the MagnetisMM-1 phase I trial evaluates elranatamab combined with other agents (i.e., dexamethasone, lenalidomide, and pomalidomide). In addition, elranatamab as monotherapy is further investigated in the MagnetisMM-3 phase II trial (NCT04649359).
By early May 2021, three cases of Grade 3 peripheral neuropathy were reported in the MagnetisMM-1 trial: one receiving elranatamab as a single agent and two in combination with pomalidomide. These AEs led to the immediate pause of patient recruitment in the phase II trial until more information was gathered.
As reported by Bahlis in the Q&A session at ASCO 2021, they are still studying the details of these events, but the peripheral neuropathy improved from Grade 3 to Grade 2 in all three patients when the treatment was discontinued. By June 2021, the sponsor was able to reinitiate patient recruitment for the MagnetisMM-3 trial safely.
For more information on neurotoxicity and anti-BCMA agents, read our editorial theme article.
Conclusion
The findings from the trial support the safety and efficacy of elranatamab at doses up to 1,000 µg/kg SC weekly in patients with RRMM. The RP2D of elranatamab is 1,000 mg/kg once a week, which achieved an ORR of 69%. All CRS events were either Grade 1 or 2 and priming and premedication reduced the incidence and duration of CRS. As a result, this two-step priming regimen has been implemented within the MagnetisMM program. Pharmacokinetics and pharmacodynamics, along with the efficacy data, also support the weekly SC dosing of elranatamab. Overall, the initial experience with elranatamab demonstrates that it is effective, achieving deep responses with a manageable safety profile in patients with heavily pretreated disease.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

