All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Efficacy and safety of CAR T-cell therapies produced in academic centers
Do you know... The CAR T-cell product ARI0002h was administered to 30 patients in its dedicated pilot study. After a median follow up of 12.1 months, what percentage of patients experienced a complete response?
Chimeric antigen receptor (CAR) T-cell therapy, an advanced therapy medicinal product, is regulated by the European Medicines Agency (EMA) as well as other federal and regional authorities.1 All products must adhere to the good manufacturing practice standards, defined as potent products manufactured safely according to standardized methods under closely controlled, reproducible, and auditable conditions.1
Historically, BioPharma has supplied the majority of CAR T-cell products; however, several academic centers have recently developed point-of-care manufacturing capabilities with the aim of improving patient access and streamlining costs.1
Two CAR T-cell products have been recently developed in academic settings, and pilot studies for each have evaluated the safety and efficacy of these potential therapies. The first product, ARI0002h, was investigated in the pilot CARTBCMA-HCB-01 study (NCT04309981) by Caldes et al.2 The second product, HBI0101, was evaluated in a phase I study (NCT04720313) by Asherie et al.3 Here, we summarize the key results from both trials.
ARI0002h2
Study design
This was a single-arm multicenter, open-label pilot study. The eligibility criteria, dosing schedule, and study endpoints were reported previously by the Multiple Myeloma Hub.
Results
A total of 30 patients with relapsed/refractory multiple myeloma received a CAR T-cell infusion, and their baseline characteristics are shown in Table 1.
Table 1. Baseline patient characteristics*
|
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System. |
|
|
Characteristic, % (unless otherwise stated) |
N = 30 |
|---|---|
|
Age, years |
61 |
|
Sex |
|
|
Male |
60 |
|
Female |
40 |
|
Median time since diagnosis, years |
4.7 |
|
ISS stage |
|
|
I |
20 |
|
II |
32 |
|
III |
48 |
|
ECOG Performance Status |
|
|
0 |
62 |
|
1 |
31 |
|
2 |
7 |
|
High-risk cytogenetics |
33 |
|
TP53 alterations |
23 |
|
t(4;14) |
13 |
|
t(14;16) |
3 |
|
Previous lines of therapy |
|
|
Triple exposed |
100 |
|
Triple refractory |
67 |
|
Penta exposed |
37 |
|
Penta refractory |
23 |
|
Refractory to the last line |
100 |
- All patients received Fractions 1 and 2.
- 86% of patients received the booster dose.
- In 19 of the 24 patients who were reinfused, 3 × 10⁶ CAR-positive cells per kg were available for the booster dose.
- Overall, 1.8 × 10⁶ CAR-positive cells per kg were available for 3 patients, and 1.2 × 10⁶ CAR-positive cells per kg were available for 2 patients.
- The median manufacturing time for ARI0002h was 10 days (interquartile range [IQR], 9–10 days).
- The mean transduction rate was 56%.
- All except one of the final CAR T-cell products were successfully obtained on the first attempt.
- The median time from apheresis reception to product liberation was 30 days (IQR, 26–36 days; range, 19–45 days).
- For patients requiring urgent treatment, the product was released in as little as 19 days.
- The overall response rate in the first 100 days post infusion was 100% (Figure 1).
Figure 1. Patient response rates in the first 100 days after ARI0002h infusion*
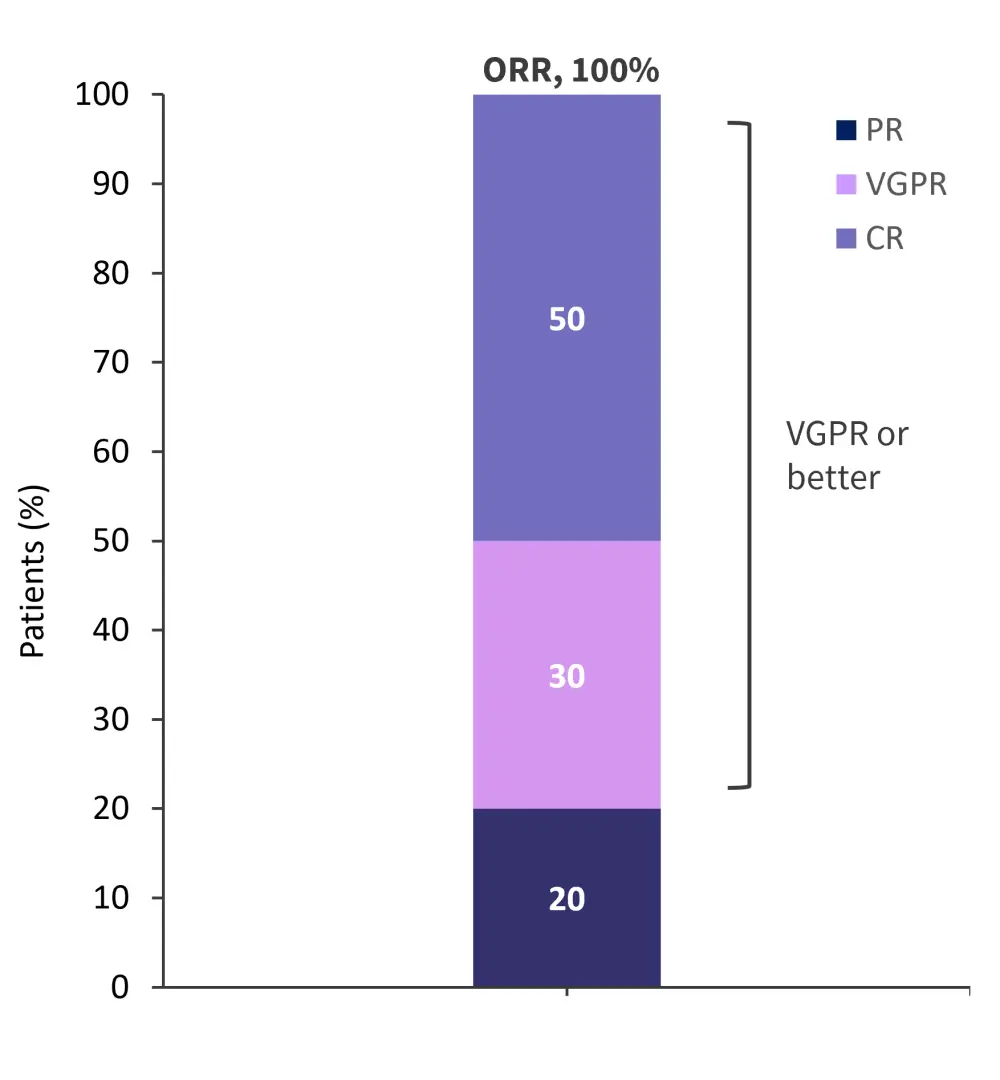
CR, complete response; ORR, overall response rate; PR, partial response; VGPR, very good partial response.
*Adapted from Caldes, et al.2
- The median time to complete response was 3.8 months (IQR, 1.0–11.6 months).
- Measurable residual disease was evaluable in 73% of patients, of which 95% experienced a negative result.
- A post hoc analysis, with a median follow-up of 18 months, showed response rates had deepened over time (Table 2)
Table 2. Response rates from the post hoc analysis*
|
*Adapted from Caldes, et al.2 |
|
|
Response, % |
N = 30 |
|---|---|
|
Overall response |
100 |
|
Complete response |
67 |
|
Very good partial response |
27 |
|
Partial response |
7 |
- The median progression-free survival was 14.5 months (95% confidence interval [CI], 12.8–not reached).
- Median overall survival and duration of response were not reached (95% CI, 8.0–not reached and 95% CI, 12.9–not reached, respectively).
- During an interim analysis at a median follow-up of 12.1 months, 33% of patients had discontinued treatment.
- Of these, 80% experienced disease progression and the remainder died without progression.
- There were no treatment discontinuations due to manufacturing failures.
- Cytokine release syndrome (CRS) was experienced by 80% of patients
- There were no Grade ≥3 events
- The median time to onset was 7 days (IQR, 5–8 days).
- The median duration of symptoms was 2 days (IQR, 0–14 days).
- There were no cases of immune effector cell-associated neurotoxicity syndrome or other late neurotoxic effects.
- There was one mild infusion reaction and one case of moderate tumor lysis syndrome.
- The most common Grade ≥3 adverse events experienced by patients are shown in Table 3.
Table 3. All Grade ≥3 adverse events*
|
*Adapted from Caldes, et al.2 |
|
|
Adverse event (%) |
N = 30 |
|---|---|
|
Anemia |
10 |
|
Neutropenia |
70 |
|
Thrombocytopenia |
47 |
|
Pyrexia |
7 |
|
Lymphocytosis |
3 |
|
Lymphopenia |
3 |
|
Febrile neutropenia |
3 |
|
Diarrhea |
3 |
|
Alanine aminotransferase increased |
3 |
|
Aspartate aminotransferase increased |
3 |
|
Leishmaniasis |
3 |
|
Rhinovirus infection |
3 |
|
Septic shock |
3 |
|
Severe acute respiratory syndrome |
3 |
|
Staphylococcal bacteremia |
3 |
|
COVID-19 |
3 |
|
Head injury |
3 |
|
Seizure |
3 |
|
Acute kidney injury |
3 |
HBI01013
Study design
This was a single-center, phase I study. The eligibility criteria, dosing schedule, and study endpoints are shown in Figure 2.
Figure 2. Eligibility criteria, dosing schedule, and study endpoints*
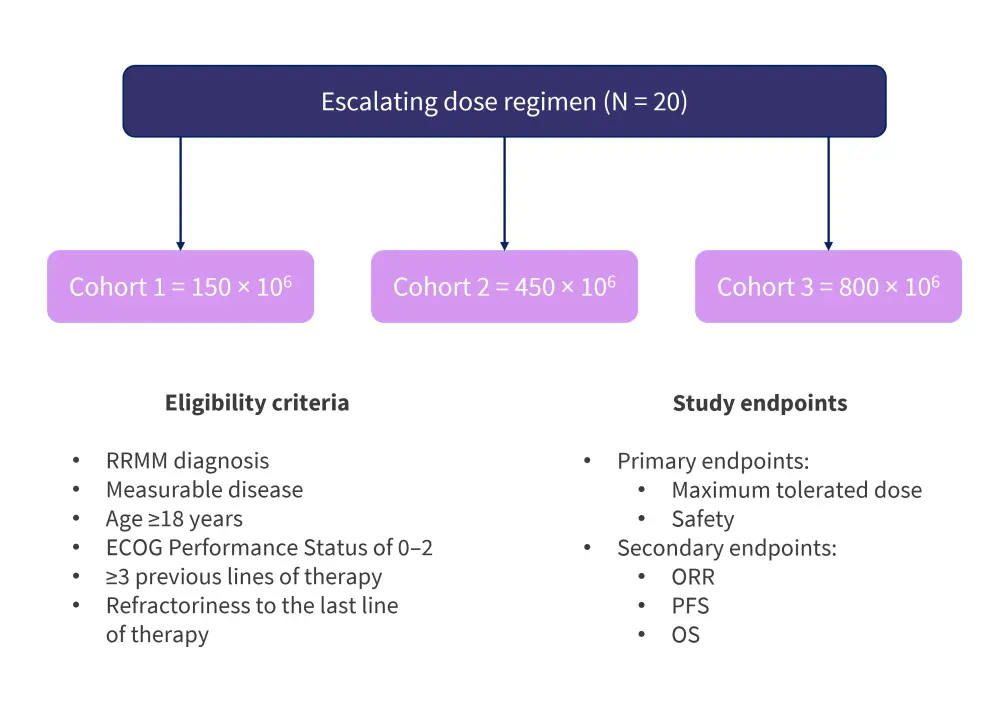
ECOG, Eastern Cooperative Oncology Group; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; RRMM, relapsed/refractory multiple myeloma.
*Adapted from Asherie, et al.3
Results
A total of 20 patients with relapsed/refractory multiple myeloma received a CAR T-cell infusion. Baseline characteristics are shown in Table 4.
Table 4. Baseline patient characteristics*
|
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System. |
|
|
Characteristic, % (unless otherwise stated) |
N = 20 |
|---|---|
|
Median age, years |
62 |
|
Sex |
|
|
Male |
40 |
|
Female |
60 |
|
Median time since diagnosis, months |
55 |
|
R-ISS |
|
|
I |
5 |
|
II |
55 |
|
III |
10 |
|
ECOG Performance Status |
|
|
0 |
35 |
|
1 |
20 |
|
2 |
45 |
|
Cytogenetic abnormalities |
|
|
High risk |
50 |
|
Standard risk |
60 |
|
Unknown |
5 |
|
Median previous lines of therapy, n |
6 |
- Drug products were successfully generated from fresh (n = 19) and cryopreserved (n = 1) raw materials
- There were no production failures, and product manufacturing was successful for all patients
- Three patients received bridging therapy during the manufacturing period
- A total of 18 patients underwent lymphodepletion
- All patients were infused with fresh drug product after 10 days of production
- The median duration of hospitalization post infusion was 17 days
- Most patients were discharged after a maximum of 25 days
- The median follow up was 136 days
- The overall response rate was 75% (Figure 3)
- 50% in Cohort 1, and 85% in Cohorts 2 and 3
Figure 3. Response rates for patients treated with HBI0101*
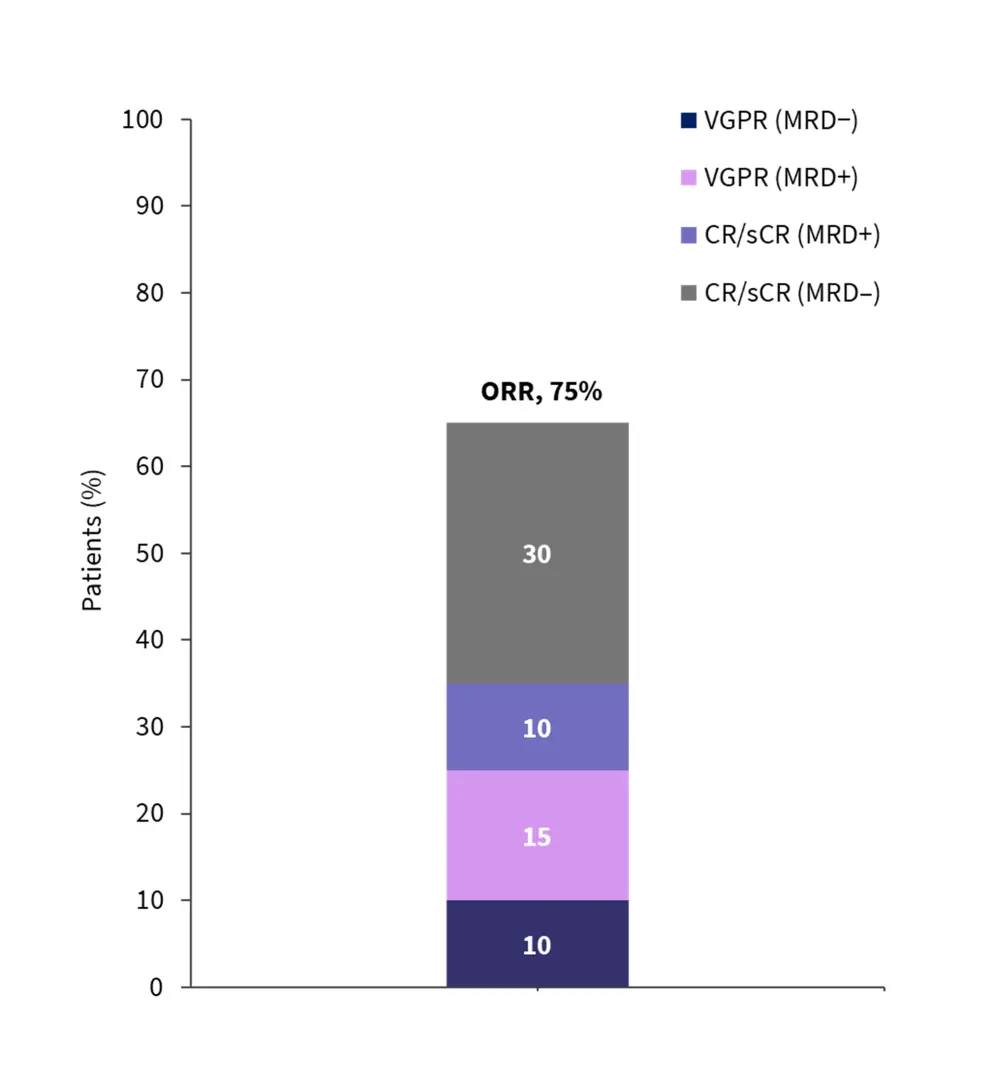
CR, complete response; sCR, stringent complete response; MRD, measurable residual disease; ORR, overall response rate; VGPR, very good partial response.
*Adapted from Asherie, et al.3
- The best response was achieved 1 month post infusion
- The median progression-free survival was 160 days
- The median overall survival was 308 days
- All Grade 3 and 4 adverse events experienced by the total patient population are shown in Table 5
Table 5. All Grade 3 and 4 adverse events*
|
*Adapted from Asherie et al.3 |
|
|
Adverse event, % |
N = 20 |
|---|---|
|
Hematologic ≤28 days |
|
|
Neutropenia |
100 |
|
Thrombocytopenia |
60 |
|
Anemia |
65 |
|
Lymphopenia |
100 |
|
Febrile neutropenia |
75 |
|
Hematologic >28 days |
|
|
Neutropenia |
30 |
|
Thrombocytopenia |
35 |
|
Lymphopenia |
30 |
|
Hypogammaglobulinemia |
25 |
|
Other ≤28 days |
|
|
Elevated liver enzymes |
10 |
|
Sepsis |
15 |
|
Infectious gastroenteritis |
5 |
|
Pulmonary edema |
5 |
|
Other >28 days |
|
|
Atrial fibrillation |
5 |
|
Pulmonary edema |
5 |
|
Elevated liver enzymes |
5 |
|
Pulmonary embolism |
5 |
CRS of any grade was experienced by 90% of patients. The median duration was 2 days, and there were no Grade ≥3 events; however, a higher rate of Grade 2 CRS was noted in Cohorts 3 and 2 compared with Cohort 1 (Figure 4).
Figure 4. Rate of CRS in Cohorts 1, 2, and 3*
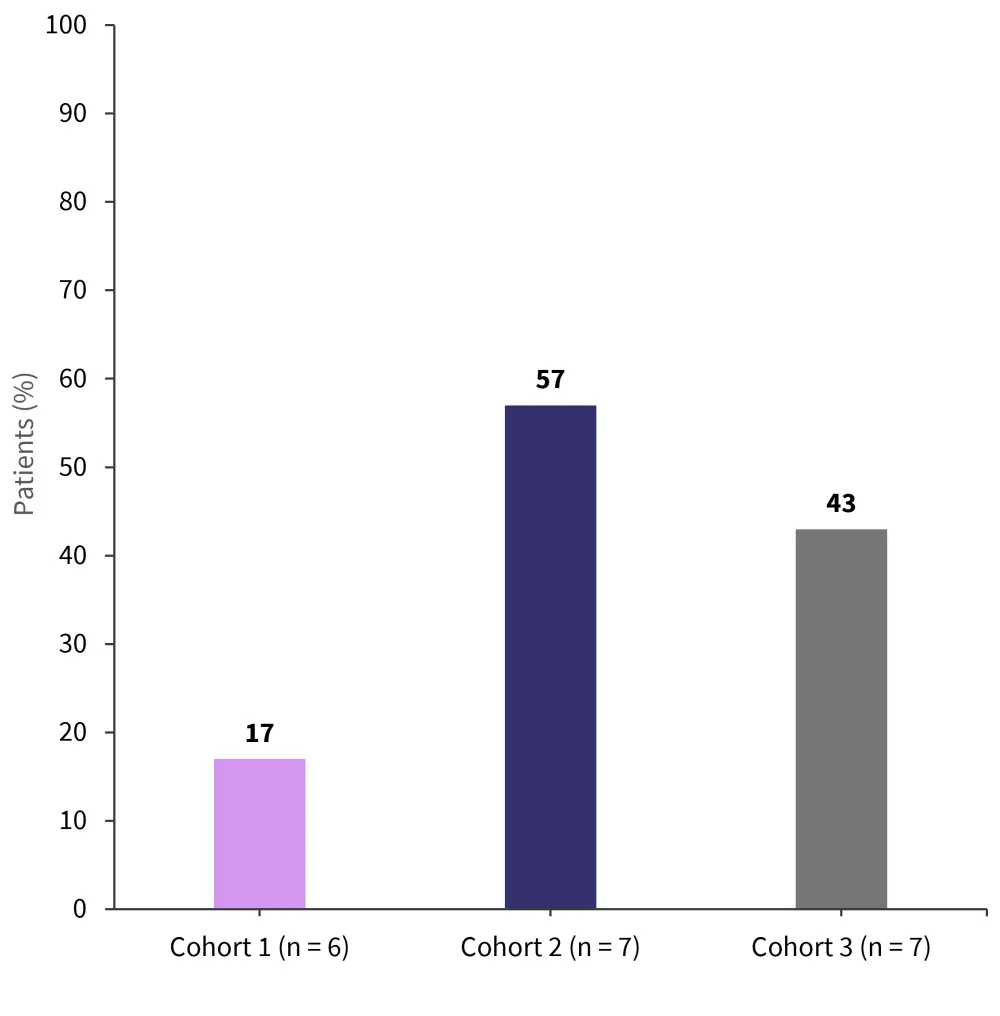
*Adapted from Asherie, et al.3
- There were no immune effector cell-associated neurotoxicity syndrome events.
- There were no treatment-related mortalities, though eight patients died due to disease progression and one died due to COVID-19.
Conclusion
Overall, both products demonstrated deep and sustained responses in patients with heavily relapsed or refractory multiple myeloma. Both the response rates and safety results were in line with those of currently approved therapies, as well as results reported in the literature. However, the relatively short median follow-up time of 136 days from the first study was cited as a limitation, as well as a small sample size; this led to wide 95% CIs in the second study. While access to CAR T-cell therapy remains limited due to logistical and financial constraints, these two academically developed products highlight the potential in broadening access for patients by offering reduced vein-to-vein time, greater manageability, and reduced financial burden, while simultaneously testifying to the capability of academic institutions in producing this advanced medicinal technology. The requirement of local current good manufacturing practice facilities and trained staff remains an overall barrier; however, the introduction of semi-automated manufacturing platforms has the potential to standardize the process across multiple sites.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




