All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Editorial theme | Peripheral neuropathy
The Multiple Myeloma Hub is pleased to present an editorial review of the management of peripheral neuropathy (PN) related to novel agents used for the treatment of multiple myeloma (MM). The Multiple Myeloma Hub has previously covered anti-B-cell maturation antigen-related neurotoxicity and recently hosted a symposium on the management of pain in patients with myeloma, including a discussion of PN (by Heinz Ludwig) and neuropathic pain (by Flaminia Coluzzi).
Peripheral neuropathy in patients with multiple myeloma
MM is characterized by the clonal proliferation of malignant plasma cells in the bone marrow and the accumulation of monoclonal protein in the blood, leading to multiple organs and tissue injury.1 Though still incurable, the treatment of patients with MM with novel agents, such as bortezomib, carfilzomib, and ixazomib, and the repurposing of old therapies, such as thalidomide, has significantly improved disease management and survival outcomes.1
These drugs currently form the backbone of MM therapy in combination with other agents. Nonetheless, toxicities and side effects can lead to a significant reduction in a patient's health-related quality of life (HRQoL), including loss of physical function, fatigue, pain, anxiety, and depression.1
Chemotherapy-induced PN is experienced in many cancer types and in MM it is mainly associated with the proteasome inhibitor bortezomib and the immunomodulatory agent thalidomide.1 PN affects the sensory, motor, and autonomic nervous systems, with sensory symptoms the most common, with varying nature (including pain, numbness, tingling, and burning) and type (including shooting, chronic, and electric shock).1 Symptoms and effects can be progressive and lead to ambulatory difficulties and loss. Without adequate management, PN and the physical sequelae can become permanent or limit treatment, negatively impacting clinical outcomes and survival.1
Bortezomib-induced peripheral neuropathy
Presentation and mechanisms
Bortezomib-induced PN (BIPN) is common and a dose-limiting non-hematologic adverse effect of MM treatment often requiring dose modification, treatment delay, or discontinuation.2 BIPN negatively impacts HRQoL with significant economic and social impact and increasing treatment costs.1,2
Patients with BIPN experience painful sensory neuropathy, paresthesia and numbness commonly presenting in characteristic symmetric "glove and stocking" distribution, and neuropathic pain, primarily affecting the fingertips and toes. Symptoms can also include autonomic dysfunction, such as orthostatic hypotension, postural dizziness, syncope, diarrhea, paralytic ileus, and urinary disturbances.2 The parasympathetic and sympathetic nervous dysfunction seen may result from toxicity to the pre- and post-ganglionic autonomic nerve fibers because of treatment with bortezomib.2 Bortezomib has also been associated with progressive and potentially significant motor neuropathy.2
The mechanisms of BIPN are not yet fully established but are thought to include aerobic glycolysis in sensory neurons and subsequent sensitization of primary afferents nerves, oxidative stress, endoplasmic reticular stress, and mitochondrial dysfunction leading to nerve cell dysfunction, symptoms, apoptosis, cytoskeletal alteration, and cytokine dysregulation (Figure 1).3,4
Figure 1. Mechanism of bortezomib-induced peripheral neuropathy*
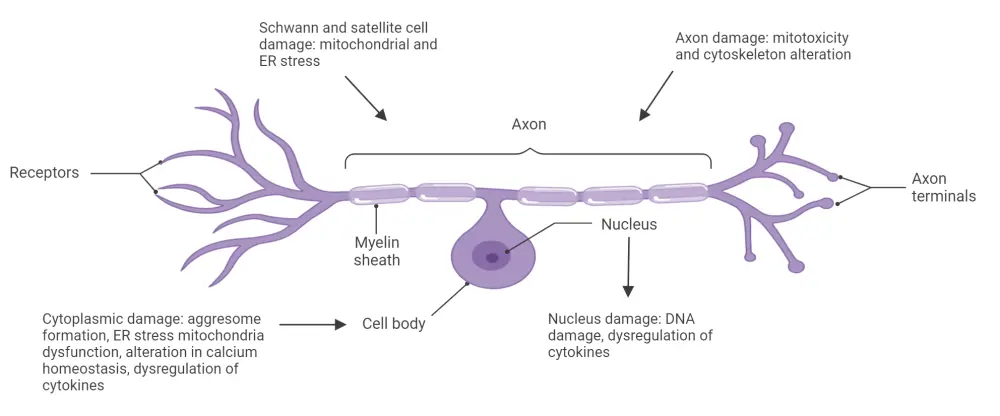
DNA, deoxyribonucleic acid; ER, endoplasmic reticulum.
*Adapted from Grammatico, et al.4 Created with BioRender.com.
Incidence and clinical considerations of bortezomib-induced peripheral neuropathy
A retrospective analysis of 8,218 patients with MM in phase III trials treated with bortezomib showed an overall incidence of PN (all grades) of 8.4–80.5% (median 37.8%) and of severe PN (Grades 3 or 4) of 1–33.2% (median, 8%).2 The incidence and the severity of the neuropathy were comparable with newly diagnosed MM (NDMM) and relapsed MM.2 There were dose reductions due to neurotoxicity in 12% and discontinuation in 5% of the patients.2
Predisposing factors for BIPN have been identified and include2:
- Pre-existing neuropathy
- Combination with thalidomide
- Advanced age
- Diagnosis of MM (patients with NDMM present with higher rates of neurotoxicity than many other malignancies)
The incidence and severity of BIPN are linked to the dose received, dosing schedule, and route of administration.2,3 Neurotoxicity is more likely to develop within the first cycles of standard-dose treatment/regimen and plateau following five 3-week cycles (at 42 mg/m2) in relapsed MM or following three 6-week cycles (at 45 mg/m2) in NDMM.2
Weekly subcutaneous bortezomib administration is associated with a significantly lower rate of neuropathy than twice-weekly intravenous administration. This is without a reduction in efficacy and with no recurrence of neurotoxicity following initial withdrawal in recovered patients who are retreated. Dose reduction, alteration in the route or schedule of dose, or treatment withdrawal can improve or resolve the neuropathy.2
The median time to recover from BIPN has been reported to be ~3 months. However, neurotoxicity continues to affect patients after treatment has finished. Up to 30% of patients can be left with persistent neuropathy and disability.2
Bortezomib-related peripheral neuropathy in clinical trials
The phase III BOSTON trial (NCT03110562) compared a triplet regimen of once per week of oral selinexor with bortezomib and low-dose dexamethasone (XVd) versus twice per week bortezomib plus dexamethasone (Vd) in patients with relapsed MM who received 1–3 prior treatments.1 This is the first trial of a bortezomib-based triplet that demonstrated lower rates of overall and Grade ≥ 2 PN compared with a Vd regimen.1 The results showed that patients achieved longer progression-free survival and required 37% fewer clinic visits than patients on a twice-weekly Vd schedule.1
Patient-reported PN was assessed using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire for Chemotherapy-Induced Peripheral Neuropathy (QLQ-CIPN20), which is a validated instrument (secondary HRQoL endpoint).1 Additionally, the EORTC Quality of Life Questionnaire for Cancer-30 item (QLQ-C30) tool was used to compare patients randomized to the XVd arm versus the Vd arm (Table 1).
Table 1. Model-based change from baseline to Day 106 on select EORTC QLQ-CIPN20 and QLQ-C30 scales from the BOSTON trial*
|
CI, confidence interval; EORTC, European Organization for Research and Treatment of Cancer; QLQ-30, Quality of Life Questionnaire for Cancer-30 item; QLQ-CIPN20, Quality of Life Questionnaire for Chemotherapy-Induced Peripheral Neuropathy; QoL, quality of life; SE, standard error; Vd, bortezomib and dexamethasone; XVd, selinexor, bortezomib, and dexamethasone. |
||||
|
Domain |
Least square adjusted mean change (SE) |
XVd vs Vd |
||
|---|---|---|---|---|
|
XVd |
Vd |
Difference |
p value |
|
|
QLQ-CIPN20 |
||||
|
Sensory |
1.59 (1.312) |
6.93 (1.265) |
−5.34 (−8.39 to −2.29) |
<0.001 |
|
Motor |
3.07 (1.247) |
4.88 (1.203) |
−1.81 (−4.72–1.10) |
0.223 |
|
Autonomic |
12.39 (1.815) |
7.40 (1.746) |
4.99 (0.73–9.25) |
0.022 |
|
QLQ-C30 |
||||
|
Pain |
−4.40 (1.994) |
2.18 (1.928) |
−6.58 (−11.36–1.80) |
0.007 |
|
Physical function |
−3.37 (1.671) |
−5.59 (1.612) |
2.22 (−1.66–6.11) |
0.262 |
|
Role function |
−9.03 (2.284) |
−7.09 (2.213) |
−1.93 (−7.38–3.52) |
0.487 |
|
Global health/QoL |
−3.45 (1.614) |
−3.13 (1.544) |
−0.32 (4.15–3.50) |
0.868 |
The phase II SUMMIT (NCT03934866) and CREST clinical trials and the phase III APEX trial reported on BIPN rates.5 SUMMIT and CREST assessed the incidence, characteristics, and reversibility of PN in patients treated with bortezomib.5 In the pooled analysis of SUMMIT/CREST trials, PN was seen in 35% of all patients. PN was also dose-dependent, reported in 37% and 21% of the patients treated with 1.3 mg/m2 and 1 mg/m2, respectively.5
The incidence of Grade 1 or 2, 3, and 4 PN was 22%, 13%, and 0.4%, respectively.5 A reduction in dosage was required in 12% of the patients and 5% discontinued treatment. Similarly, patients who developed PN were able to reach a complete resolution or improved during follow-up (in 51% of the patients with Grade ≥2 PN and in 71% of the patients with Grade 3−4 PN or with PN resulting in treatment discontinuation).5 The median time to improvement or resolution of the PN was 47 days (range, 1–529 days).5 The majority of patients with PN in the SUMMIT/CREST trials did not report an aggravated PN with protracted treatment, suggesting prolonged exposure to bortezomib does not increase the incidence or severity of the PN.5
In the APEX (NCT00048230) clinical trial, 37% of patients allocated to bortezomib reported BIPN, including 27% with Grade ≥2, 9% with Grade ≥3, and <1% with Grade 4. Most cases of PN were sensory and only a few were motor. A total of 64% of patients with Grade ≥2 PN had an improvement or resolution to baseline at a median of 110 days (range, 4–627) days, including 68% who had dose modification compared with 47% who did not. Additionally, the efficacy was not significantly reduced by dose modification for Grade ≥2 PN. The incidence and severity of PN were not affected by age, number/type of prior therapies, baseline glycosylated hemoglobin level, or diabetes history.
In the phase III VISTA trial, peripheral sensory neuropathy was more common in the bortezomib group, including Grade 1 neuropathy in 14%, Grade 2 in 17%, Grade 3 in 13%, and Grade 4 in <1% of patients. A total of 74% of PN events had either resolved (56%) or reduced by at least one toxicity grade (18%) within a median of two months.
Thalidomide-induced peripheral neuropathy
Presentation and mechanisms
Thalidomide-induced PN (TIPN) manifestations include bilateral and symmetrical sensory changes similar to BIPN, but with less frequent motor or autonomic symptoms or signs.5 Distal paresthesia and hyperesthesia affecting the toes more than fingers is seen, with occasional proximal extension. The most common motor manifestation is tremor, with limited functional impact in the early stages.5 Loss of proprioception can occur with intention tremor, progressive ataxic gait, and eventual difficulty walking.5
The mechanisms underlying TIPN are also unclear but are thought to arise from the reduced nerve blood supply, dysregulation of neurotrophins, and Wallerian degeneration (axon degeneration distal to the point of injury). There is thought to be a pharmacogenetic predisposition to TIPN (Figure 2).5,6
Figure 2. Mechanisms of thalidomide-induced peripheral neuropathy*
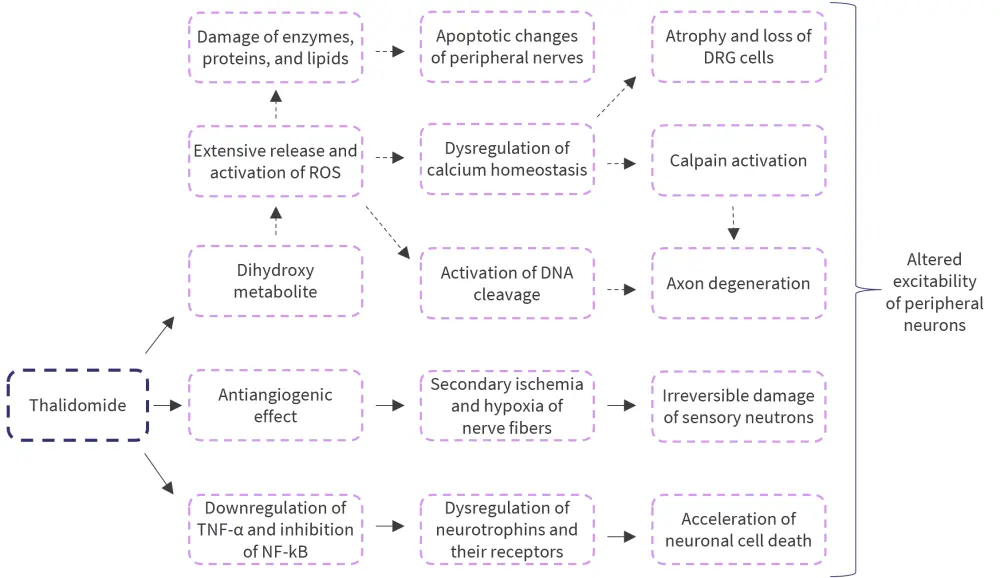
DNA, deoxyribonucleic acid; DRG, dorsal root ganglia; NF-kB, nuclear factor kappa B; ROS, reactive oxygen species; TNF-α, tumor necrosis factor α.
*Adapted from Zajaczkowska, et al.6
Incidence and clinical considerations of thalidomide-induced peripheral neuropathy
The incidence of TIPN varies according to different studies given the differences in assessment, rating criteria, diagnosis, and short follow-up periods, but it ranges from 25% to 83%, with treatment interruption in about 15% of the patients.5 The incidence of TIPN has been identified as higher in patients aged >65 years (41% vs 17%; p < 0.001; but this had not been replicated in other studies).5
The relationship between the duration of exposure, dose intensity, and cumulative dose of thalidomide on the incidence of TIPN remains unclear.5 High cumulative dose has been associated with increased risk of TIPN, with aggravation of TIPN in patients given higher doses.5 Conflicting findings regarding cumulative dose and TIPN risk have been found in clinical trials and low-dose thalidomide treatment is thought to reduce the risk of TIPN, with incidence with doses <150–200 mg/day.5 While low doses may reduce TIPN risk and be better tolerated by patients in terms of other adverse events, the risk of long-term neurotoxicity remains.
Management of bortezomib and thalidomide-induced neuropathy: treatment and guidelines
BIPN and TIPN cannot be prevented with no current risk-stratification strategies.3,5,6 Treatment options are limited to symptomatic therapies that aim to reduce neuropathic pain3, holistic care including physiotherapy and psychology (covered on the MM Hub here), and alternative therapies (including acupuncture).3 Systemic pharmacologic management includes tricyclic antidepressants, duloxetine, and gabapentinoid, with focal pain treated topically with lidocaine or high-dose capsaicin patches. Furter details are discussed here.3
In the clinical trials considered above, the lower frequency of Grade ≥3 BIPN seen in the APEX trial (compared with SUMMIT/CREST) was credited to the investigator's knowledge of BIPN, the use of dose-modification guidelines, and ability to utilize them effectively (Figure 3). Clinical guidelines are essential to guide effective drug dose adjustment to allow for the resolution of PN while minimizing loss of dosing and treatment efficacy.
Figure 3. Guidelines for the management of bortezomib and thalidomide-induced PN evaluated according to the NCI CTCAE*
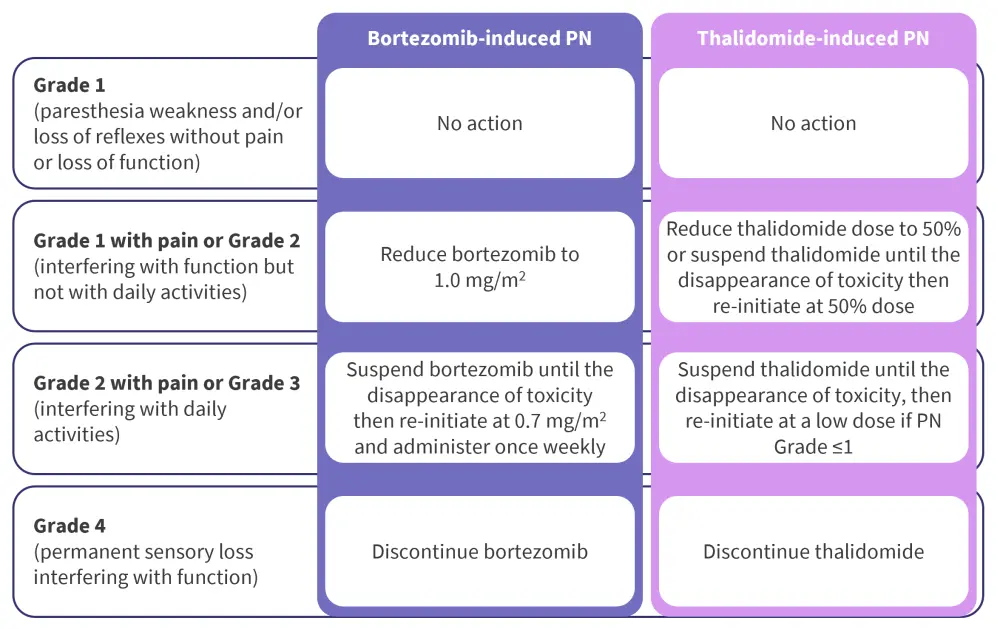
NCI CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events; PN, peripheral neuropathy.
*Adapted from Grammatico, et al.4
Conclusion
While MM remains incurable, treatments like bortezomib and thalidomide have significantly improved clinical outcomes. However, PN remains a significant adverse event, with management a major challenge. Further studies are warranted to assess risk factors of PN including the need for a simple, widely usable, and effective grading system. Early detection of PN and dose adjustment algorithms or modification of administration schedules should help decrease the side effects incidence while maintaining treatment efficacy. In addition, identifying pharmacogenomic and clinical risk factors may allow for improved risk stratification, treatment personalization, and dose adjustment while strategies to prevent treatment-induced PN remain an area of significant unmet need.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

