All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Dual antigen targeting immunotherapies in MM
Immune-based therapies in multiple myeloma (MM) include agents such as chimeric antigen receptor (CAR) T cells, as well as bispecific antibody therapies. These novel agents are often associated with high initial response rates compared with standard therapies, yet most patients go on to experience a relapse. In an effort to improve duration of response, dual antigen targeting and trispecific antibodies are currently being investigated in MM.1
Here we summarize a presentation by Sham Mailankody1 from the International Myeloma Society’s 5th Immune Effector Cell Therapies in Multiple Myeloma Workshop, 2024, on antigen-targeting CAR T cells and antibody therapies in MM.
Dual antigen-targeting1
- The high incidence of relapse after immune therapies can be attributed to a multitude of resistance mechanisms; most commonly reduction or loss of the target antigen.
- Immune therapies such as CAR T cells and bispecific antibodies are often administered sequentially after relapse. However, use of these therapies in combination to target more than one antigen simultaneously could be applied to further reduce malignant cells and extend duration of response.
- In a mouse model, dual targeting of B-cell maturation antigen (BCMA) and G protein-coupled receptor class C group 5 member D (GPRC5D) resulted in a more durable response than either monotherapy in isolation.
- Dual antigen targeting can be achieved using combinations of CAR T-cell and bispecific antibody therapies (Figure 1).
Figure 1. Methods of dual antigen-targeting in MM*
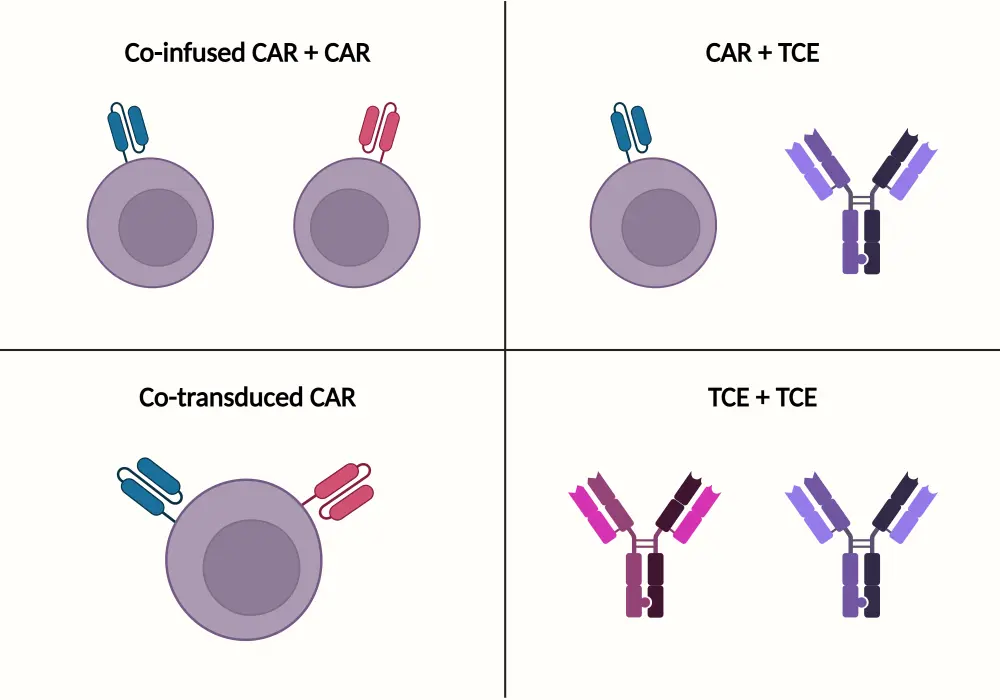
CAR, chimeric antigen receptor; TCE, T-cell engager.
Created with BioRender.com.
*Adapted from Mailankody.1
RedirecTT-11
- RedirecTT-1 (NCT04586426) is a phase I/II clinical trial investigating the combination of teclistamab and talquetamab to simultaneously target BCMA and GPRC5D for the treatment of relapsed/refractory MM.
- The trial design and latest data from RedirecTT-1 have been reported by the Multiple Myeloma Hub here.
- Data collected from this trial provide evidence to support dual antigen targeting as a strategy to aid improve response rates, particularly in difficult-to-treat presentations such as extramedullary disease (EMD) (Figure 2).
Figure 2. Response rates from RedirecTT-1 in A response-evaluable patients and B patients with EMD*
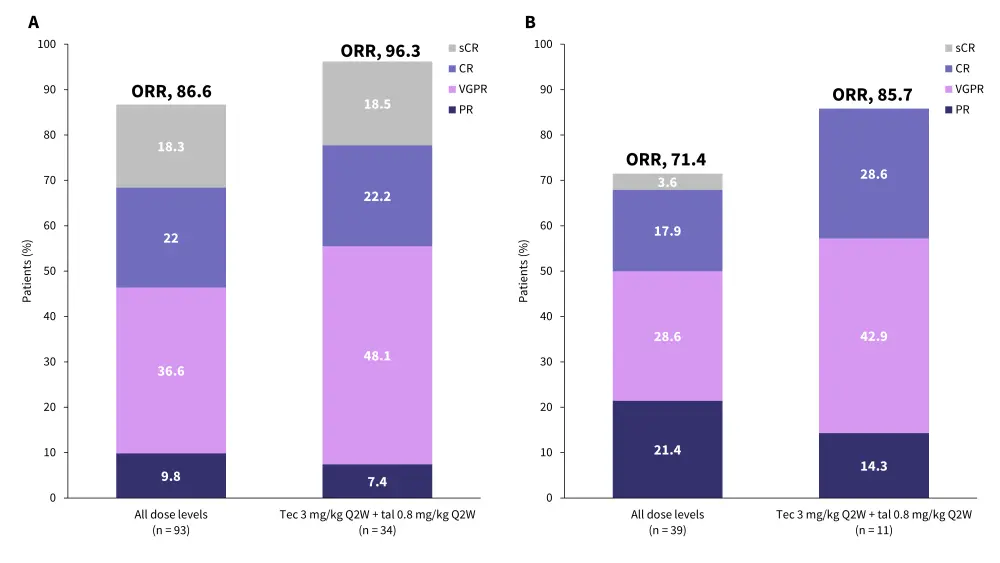
CR, complete response; PR, partial response; Q2W, once every 2 weeks; sCR, stringent complete response; tal, talquetamab; tec, teclistamab; VGPR, very good partial response.
*Adapted from Mailankody.1
|
Key learnings1 |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




