All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Resistance mechanisms to CAR T-cell therapy
The introduction of chimeric antigen receptor (CAR) T-cell therapy for the treatment of relapsed/refractory multiple myeloma has resulted in increased complete response and measurable residual disease-negativity rates for some patients with multiple previous lines of therapy.1 However, recent data on progression-free survival have not demonstrated any clear curve plateaus, highlighting the need to better understand therapy failure and resistance mechanisms to CAR T-cell therapy.1
Resistance can occur from multiple sources, including multiple myeloma microenvironments, tumor-related factors, the patient’s T-cell characteristics, and CAR T-cell characteristics (Figure 1). Here, we summarize a lecture by Van de Donk1 on the resistance mechanisms to CAR T-cell therapy presented at the International Myeloma Society 4th Immune Effector Cell Therapies in Multiple Myeloma Workshop.
Figure 1. Mechanisms of resistance to CAR T-cell therapy*
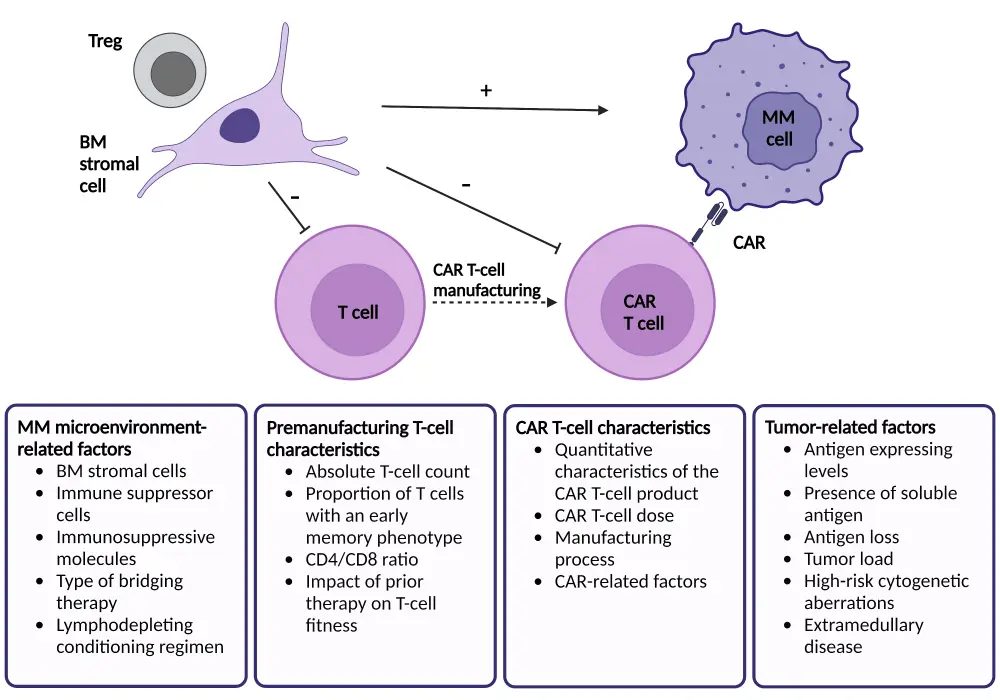
BM, bone marrow; CAR, chimeric antigen receptor; MM, multiple myeloma; Treg, regulatory T cell.
*Adapted from Van de Donk.8 Created with BioRender.com.
Antigen escape
For CAR T-cell activity, a minimum antigen expression is required; therefore, when there is a loss of antigens, the efficacy of CAR T-cell therapy is reduced.2 Following B-cell maturation antigen (BCMA) CAR T-cell therapy, there can be a reduction or even a total loss of BCMA expression in a small subset of patients. Multiple options exist to combat this antigen loss, such as using two different CAR T‑cell products concurrently or a T cell with two different binding domains for two antigens.2 This may also be addressed with the development of novel receptors that require a lower antigen density threshold, such as the chimeric costimulatory receptor CAR T cell.3 Methods to address antigen escape are outlined in Figure 2.
Figure 2. Mechanisms to combat antigen escape in CAR T-cell therapy*
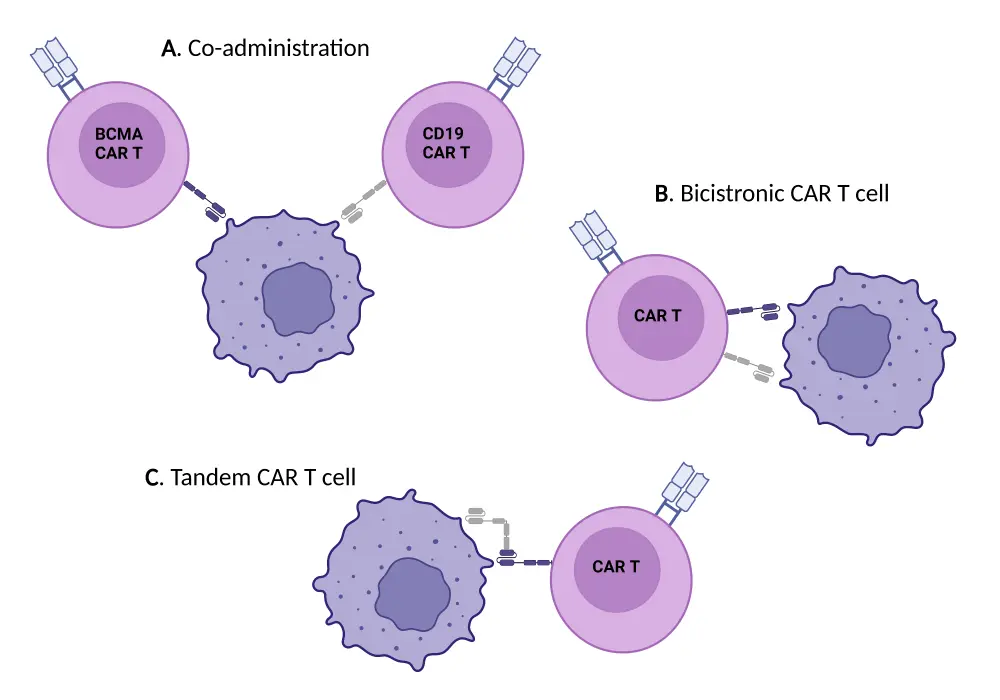
CAR, chimeric antigen receptor.
*Adapted from Rasche, et al.3 Created with BioRender.com.
Lack of persistence of CAR T cells
Multiple factors contribute to the lack of persistence of CAR T cells, one example of this being the therapies to which patients have been previously exposed.1 Patients treated with idecabtagene vicleucel who had been treated with an alkylator, proteasome inhibitors, or topoisomerase inhibitor have been found to have poorer overall progression-free survival.5 Since treatment with CAR T cells is recommended in later lines of therapy, there is a high likelihood that a patient receiving CAR T-cell therapy will have been exposed to a treatment that may negatively impact their outcomes.1
Patients with a smaller proportion of undifferentiated T cells were also found to be less likely to respond to BCMA CAR T-cell therapy.1 Methods to increase the proportion of CAR T cells produced from an immature memory T-cell phenotype may include reducing the production time or adding a phosphoinositide 3-kinase inhibitor in the culture condition. A recent example of this comes from clinical trials where BMS-986354, a less differentiated and faster-to-produce CAR T cell, was compared with its similar orvacabtagene autoleucel and was found to have superior tumor control at the same dose.6
Reduced functionality of CAR T cells
Stromal cells can suppress the efficacy of CAR T cells by upregulating anti-apoptotic molecules in the tumor cells, outlined in Figure 1.1 Designing CAR T cells that can overcome the immune suppression in the tumor environment may overcome this resistance mechanism, for example, by developing CAR T cells that produce pro-inflammatory cytokines such as IL-12. Maintenance strategies, such as lenalidomide treatment, could also be applied to mitigate the negative impacts of the bone marrow microenvironment. Lenalidomide has been shown to have positive effects on regulatory T cells and can improve CAR T-cell function, indicating its use as a maintenance therapy after CAR T-cell infusion.7
Conclusion
Improved understanding of therapy failure in CAR T cells and methods of resistance may allow for the development of more resilient and effective CAR T-cell therapies. The primary issues include the lack of persistence, antigen escape, and reduced functionality of CAR T cells. Methods to address these are being investigated, focusing on optimizing CAR design, culturing conditions during manufacturing, and amending therapy guidelines.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



