All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
ABBV-383, a novel BCMA/CD3 bispecific antibody for the treatment of RRMM
ABBV-383 (previously known as TNB383B) is a monoclonal, B-cell maturation antigen (BCMA)-targeted, IgG4 bispecific antibody.1 It features a low-activating CD3 receptor that preferentially activates effector T cells rather than regulatory cells and minimizes cytokine induction of T-cell activity.1 ABBV-383 expresses a heavy and light chain receptor pairing, which binds to CD3, and a second heavy chain, which targets BCMA.1,2
Figure 1. Structure of ABBV-383*
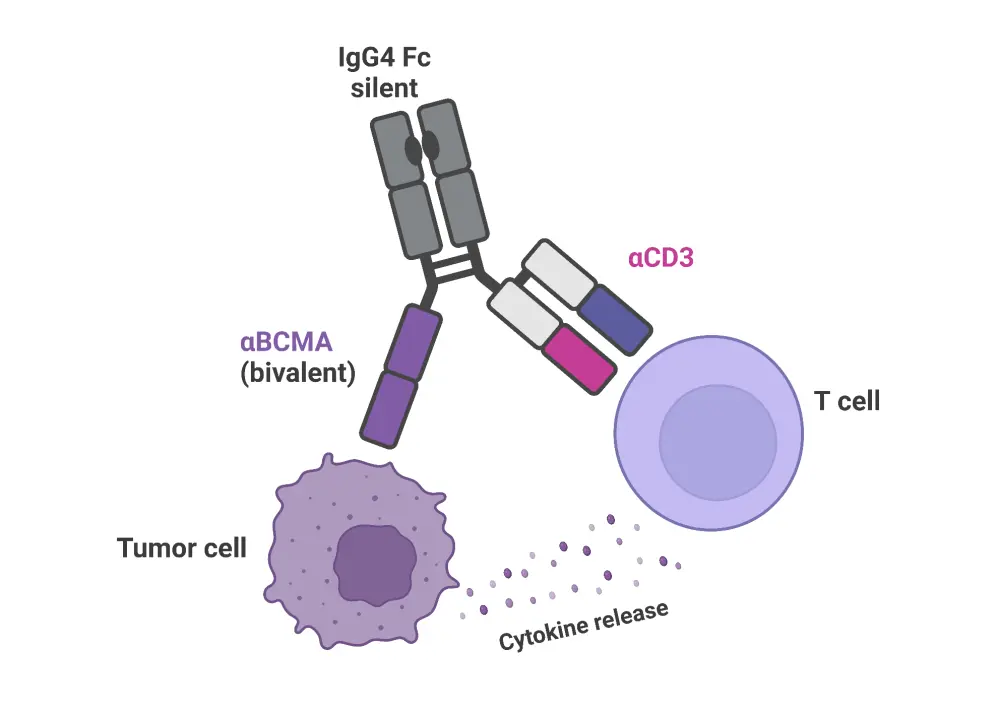
*Adapted from Abbvie.2 Created with BioRender.com.
The Multiple Myeloma Hub has previously covered the impact of BCMA therapy on the quality of life of patients after treatment, the prevalence of therapy-related cytopenias and infection following BCMA treatment, and BCMA-associated complications. It is intended that with high tumor cell avidity, but low immunogenicity, ABBV-383 will provide high efficacy for the treatment of relapsed/refractory multiple myeloma (RRMM), and be well tolerated, especially through the mitigation of severe cytokine release syndrome (CRS).1
Here, the Multiple Myeloma Hub presents data from an ongoing, open-label, phase I clinical trial (NCT03933735) investigating ABBV-383 in patients with RRMM. Data from this study were initially published by Anita D’Souza et al.1 in the Journal of Clinical Oncology in September 2022, and at the 19th International Myeloma Society Meeting, Peter Voorhies presented an update, including early data from the latest dose escalation (ESC) and expansion (EXP) phases of the study.3
Study design
The study was a phase I, multicenter, open-label clinical trial, consisting of a dose ESC phase followed by dose EXP phase. All patients were aged ≥18 years and had received ≥3 prior lines of therapy, including a protease inhibitor, an anti-CD38 monoclonal antibody, and an immunomodulatory drug. The study design can be seen in Figure 2.
Figure 2. Study design of the EBBV-383 clinical trial*
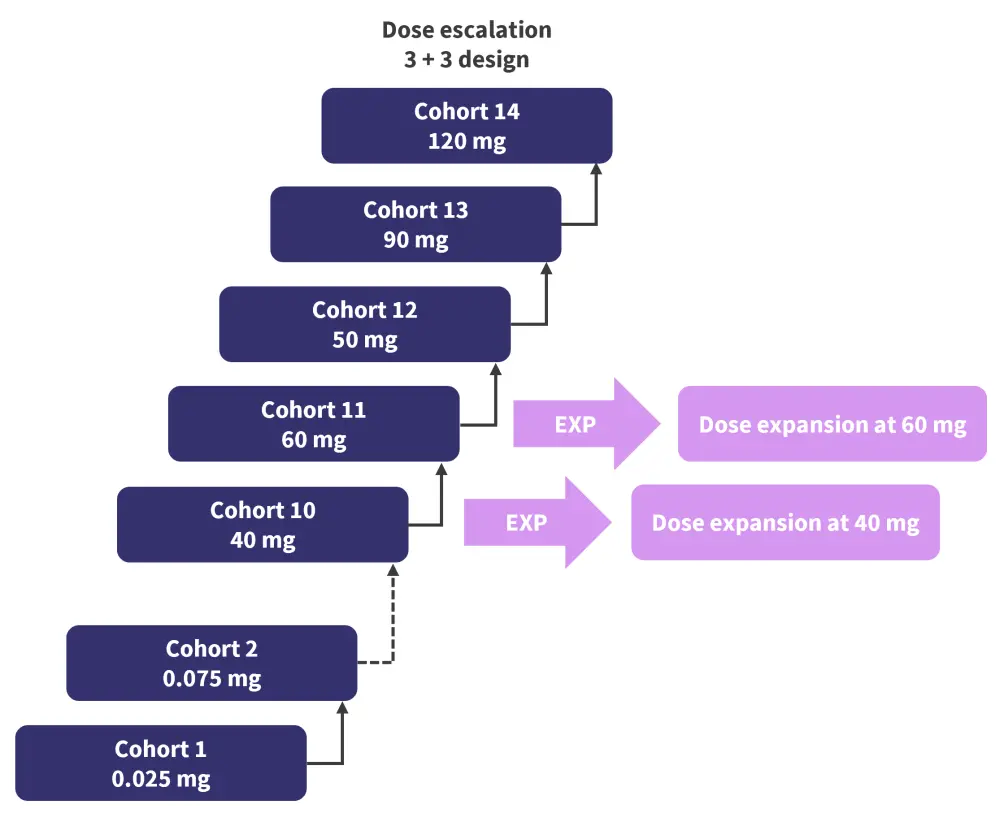
*Adapted from Voorhies.3
The primary study objectives were to investigate:
- the safety and tolerability of ABBV-383, through the evaluation of adverse events (AEs), laboratory profiles, and clinical and vital signs;
- the maximum tolerated dose; and
- the maximum dose appropriate for a phase II trial.
To investigate the clinical pharmacology of ABBV-383, blood samples for pharmacokinetic analysis were collected at defined time points throughout the study and analyzed using validated assays.
The secondary objective was to evaluate the clinical activity of ABBV-383 monotherapy using the International Myeloma Working Group (IMWG) 2016 criteria.
Results
A total of 124 patients were recruited, with 60 patients in the 60 mg ESC + EXP cohort and 81 patients in the ≥ 40mg ESC + EXP cohorts. Each patient’s first dose of ABBV-383 was given in hospital, with all other doses given as an outpatient. Patient characteristics can be seen in Table 1.
High-risk cytogenetics were defined as the presence of t(4;14), t(14;16), or 17p deletion as detected by fluorescence in situ hybridization analysis of CD138-enriched bone marrow aspirates performed at screening.z
Table 1. Patient characteristics in the EBBV-383 clinical trial*
|
ECOG PS, Eastern Cooperative Oncology Group performance status; ESC, dose escalation; EXP, dose expansion; IMiD, immunomodulatory drug; ISS, International Staging System; MM, multiple myeloma; PI, proteasome inhibitor; SCT, stem cell transplant. |
|||
|
Characteristic |
60 mg ESC + EXP cohort |
≥40 mg ESC + EXP cohort |
All patients |
|---|---|---|---|
|
Median age (range), years |
68 (35–92) |
68 (35–92) |
68 (35–92) |
|
ECOG PS score†, % |
|
|
|
|
0 |
38 |
38 |
32 |
|
1 |
53 |
54 |
57 |
|
2 |
7 |
5 |
9 |
|
Revised ISS stage at entry‡, % |
|
|
|
|
I |
17 |
17 |
15 |
|
II |
23 |
21 |
19 |
|
III |
28 |
30 |
31 |
|
Median prior lines of therapy (range) |
5 (3–12) |
4 (3–12) |
5 (3–15) |
|
Prior MM therapy, % |
|
|
|
|
Autologous SCT |
80 |
83 |
81 |
|
Double refractory§ |
87 |
84 |
84 |
|
Triple-class refractory‖ |
83 |
81 |
82 |
|
Penta-drug refractory¶ |
42 |
41 |
35 |
|
Refractory to last prior cancer therapy#, % |
85 |
85 |
87 |
An overall response rate of 57% and at least a very good partial response of 43% was seen in all patients; these rates were 60% and 43%, respectively, in the 60 mg ESC + EXP cohort, and 68% and 54%, respectively, in the ≥40 mg ESC + EXP cohort. Further outcome data, including the response rate of triple-class refractory patients, are outlined in Figure 3.
Figure 3. Response rates of the different trial cohorts*
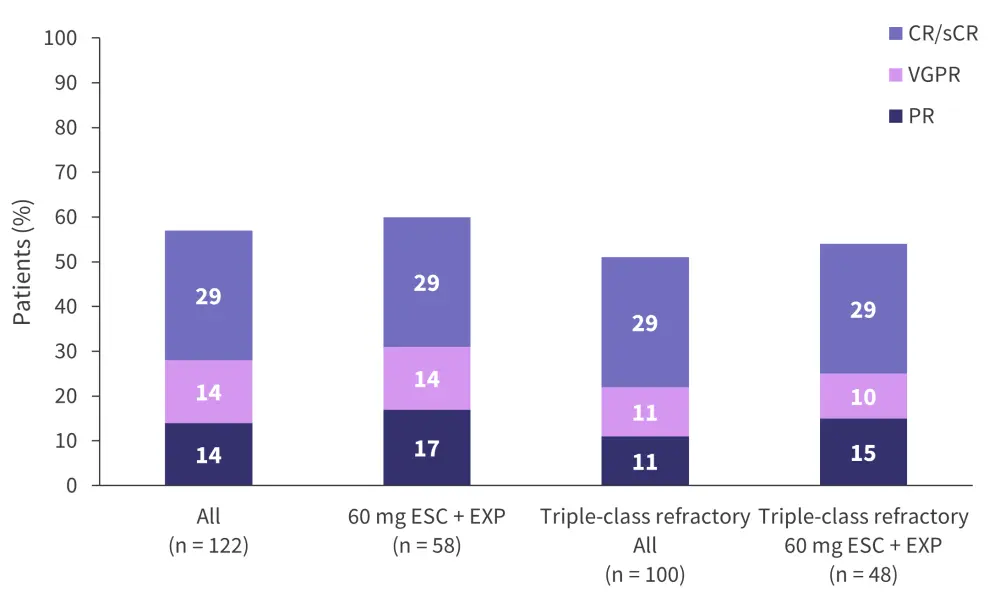
CR, complete response; PR, partial response; sCR, stringent complete response; ESC, escalation; EXP, expansion; VGPR, very good partial response.
*Adapted from Voorhies.3
Median duration of response was not reached for either the overall population or the 60 mg ESC + EXP cohort a at median follow-up of 10.8 months and 8.2 months, respectively. The median progression-free survival was 10.4 months (95% confidence interval [CI[, 5.0–19.2) for the overall cohort and not reached for the 60 mg ESC + EXP cohort. Based on Kaplan-Meier analysis, the estimated median 6- and 12-month progression-free survival rates for the 60 mg ESC + EXP cohort were 60.9% (95% CI, 44.9–73.6) and 57.9% (95% CI, 41.6–71.1), respectively, and 57.5% (95% CI, 47.3–66.4) and 46.6% (95% CI, 35.9–56.6), respectively, for the overall study cohort.
From the 60 mg ESC + EXP cohort, 45% continued to receive treatment at the time of data analysis, with a median follow-up of 8 months. No patients had been lost to follow-up. Further patient outcomes can be seen in Table 2.
Table 2. Treatment status of patients recruited to the EBBV-838 clinical trial*
|
AE, adverse event; DLT, dose-limiting toxicity; ESC, dose escalation; EXP, dose expansion. |
|||
|
Variable, % (unless otherwise stated) |
60 mg ESC + EXP cohort |
≥40 mg ESC + EXP cohort |
All patients |
|---|---|---|---|
|
Median follow-up (range), months |
8 (1-17) |
9.6 (0.6–18.2) |
11 (1-28) |
|
Treatment ongoing |
45 |
47 |
36 |
|
Discontinued treatment |
55 |
53 |
64 |
|
Reasons for discontinuation |
|
|
|
|
Disease progression |
38 |
38 |
48 |
|
DLT/AE |
5 |
5 |
6 |
|
Withdrawal of consent |
5 |
5 |
4 |
|
Death |
17 |
17 |
27 |
A serious AE (defined as causing death, a life-threatening event, hospitalization, significant disability/incapacity, or medical/surgical intervention to prevent a serious outcome) occurred in 53% of the overall patient population and in 58% of the 60 mg ESC + EXP cohort. Immune effector cell-associated neurotoxicity syndrome was reported in 3 patients, all of whom had received the 60 mg dose. There were 7 patient deaths, which were all deemed not to be related to ABBV-383.
Grade ≥3 cytokine release syndrome (CRS) occurred in 2%, 4%, and 2% of the 60 mg ESC + EXP cohort, ≥40 mg ESC + EXP cohort, and all patients, respectively. CRS was mostly experienced on the first day of treatment, with rapid recovery of patients with routine medical care. Most CRS events were Grade 1 or 2 (95.8% in the overall population).
Among all patients, neutropenia and anemia were the most common any grade hematologic AE (37% and 29%. respectively) and CRS and fatigue were the most common nonhematologic AE (57% and 30%, respectively). Further information of the AEs observed and the effects on patients are shown in Tables 3 and 4.
Table 3. Prevalence of adverse events*
|
CRS, cytokine release syndrome; ESC, dose escalation; EXP, dose expansion. |
||||||
|
Adverse event, % |
60 mg ESC + EXP cohort |
≥40 mg ESC + EXP cohort |
All patients |
|||
|---|---|---|---|---|---|---|
|
Any Grade |
Grade ≥3 |
All Grades |
Grade ≥3 |
Any Grade |
Grade ≥3 |
|
|
Hematologic |
|
|
|
|
|
|
|
Neutropenia |
42 |
37 |
46 |
41 |
37 |
34 |
|
Anemia |
32 |
12 |
35 |
17 |
29 |
16 |
|
Thrombocytopenia |
25 |
12 |
25 |
11 |
23 |
12 |
|
Lymphopenia |
18 |
17 |
20 |
16 |
15 |
13 |
|
Nonhematologic |
|
|
|
|
|
|
|
CRS |
72 |
2 |
73 |
4 |
57 |
2 |
|
Fatigue |
27 |
0 |
30 |
0 |
30 |
1 |
|
Nausea |
32 |
0 |
35 |
2 |
29 |
2 |
|
Diarrhea |
28 |
2 |
32 |
1 |
27 |
2 |
|
Vomiting |
23 |
0 |
28 |
0 |
24 |
0 |
|
Pyrexia |
22 |
0 |
21 |
0 |
19 |
0 |
|
Arthralgia |
15 |
0 |
15 |
0 |
18 |
0 |
|
Cough |
22 |
0 |
20 |
0 |
16 |
0 |
|
Headache |
10 |
0 |
12 |
1 |
16 |
2 |
|
Pain in extremity |
15 |
0 |
17 |
0 |
16 |
0 |
Table 4. Effects of adverse events on patient retention*
|
DLT, dose-limiting toxicity; ESC, dose escalation; EXP, dose expansion. |
|||
|
Adverse event, % |
60 mg ESC + EXP cohort |
≥40 mg ESC + EXP cohort |
All patients |
|---|---|---|---|
|
Leading to study discontinuation |
8 |
7 |
10 |
|
Leading to dose reduction |
7 |
7 |
5 |
|
Associated with DLT |
2 |
4 |
2 |
|
Leading to death |
3 |
5 |
6 |
Conclusion
At a dose of 60 mg, ABBV-383 demonstrated good tolerability and efficacy in patients with RRMM, including those who had been heavily pretreated. While AEs occurred, ABBV-383 was well tolerated, with a low prevalence of significant CRS. Further efficacy and outcome data are anticipated at the upcoming 64th American Society of Hematology Annual Meeting and Exposition.4
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




