All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
RedirecTT-1: Talquetamab + Teclistamab in RRMM
Teclistamab is the first B-cell maturation antigen (BCMA)-directed bispecific antibody approved for the treatment of triple-class exposed, relapsed/refractory multiple myeloma (R/R MM). Talquetamab is an investigational bispecific T-cell engager antibody targeting both GPRC5D and CD3 for the treatment of patients with R/R MM. The Multiple Myeloma Hub has previously reported on talquetamab for R/R MM from the MonumenTAL trials.
Simultaneous targeting of two validated MM target antigens, with combination teclistamab and talquetamab treatment, may improve outcomes and overcome resistance mechanisms, such as antigen escape.
Cohen presented the first results from the RedirecTT-1 study (NCT04586426) of teclistamab in combination with talquetamab, simultaneously targeting BCMA and GPRC5D, in patients with R/R MM at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting.1 These are the first results of two bispecifics used in combination for a hematological malignancy. We are pleased to summarize the key findings below.
Study design
- RedirecTT-1 applied a comprehensive dose escalation approach that incorporated a step-up dosing schedule (Figure 1).
- Inclusion criteria:
- MM diagnosis per International Myeloma Working Group 2016 criteria
- R/R or intolerant to the last line of therapy
- Prior exposure to a proteasome inhibitor, immunomodulatory drug, and anti-CD38 therapy (triple-class exposed)
- Measurable disease
- Primary objectives:
- evaluate the safety of teclistamab and talquetamab
- identify a recommended phase 2 regimen (RP2R) for the combination
- Secondary objectives:
- preliminary anticancer activity of each treatment
- pharmacokinetics
- immunogenicity
Figure 1. RedirecTT-1 study design*
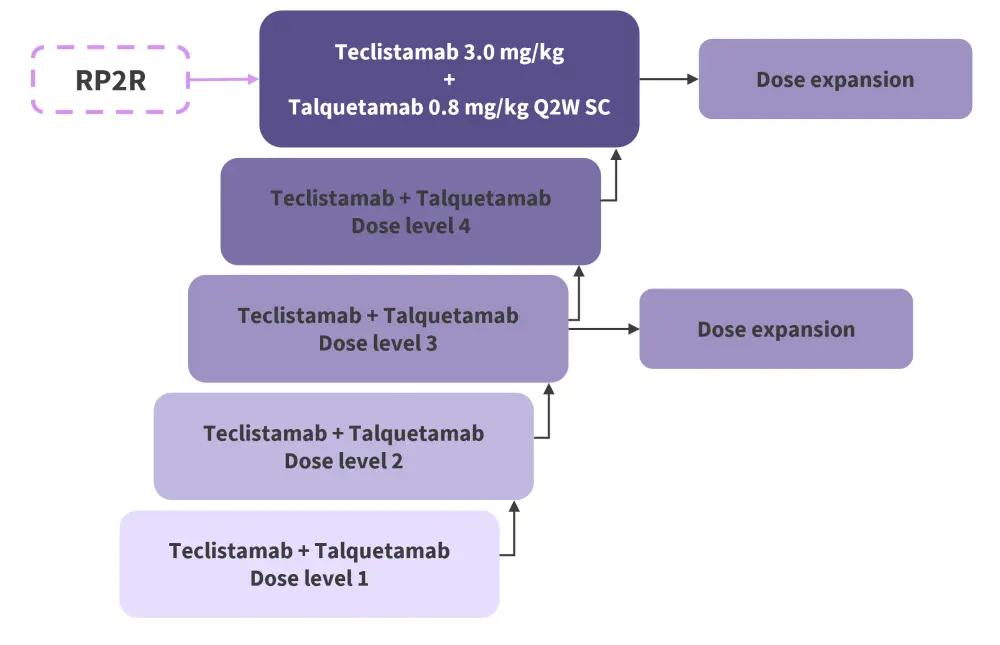
Q2W, dosing every 2 weeks; RP2R; recommended phase 2 regimen; SC, subcutaneous.
*Adapted from Cohen.1
Patient characteristics
- RedirecTT-1 enrolled 93 patients, with 34 of these treated at the RP2R dose
- Characteristics of these 34 patients were similar to those of the entire cohort
- Of 93 patients,
- 33.3% had high-risk cytogenetics, defined by the presence of del(17p), t(4;14), and/or t(14;16);
- 37.6% had extramedullary plasmacytoma(s), originating in soft tissue and measuring ≥2 cm;
- 65.5% were penta-drug exposed, including prior BCMA-targeted agents;
- 89.2% were refractory to their last line of therapy; and
- 79.6% were triple-class refractory.
- Median age was 65 years
- Median lines of prior therapy was four
Safety
Overall, the safety profile of teclistamab with talquetamab was considered clinically manageable, with low rates of discontinuation and death (Table 1). Any grade and Grade 3/4 infections occurred in 83.9% and 52.7% of patients, respectively, and all deaths and discontinuations were due to infections.
Table 1. RedirecTT-1 safety results*
|
RP2R; recommended phase 2 regimen; TEAE, treatment emergent adverse event; TRAE, treatment related adverse event. |
||
|
TEAE, % |
All dose levels |
RP2R Dose† |
|---|---|---|
|
Any TEAE |
96.8 |
94.1 |
|
Grade 3/4 TEAE |
88.2 |
79.4 |
|
Discontinuation due to drug-related TEAE |
6.5 |
5.9 |
|
Death due to drug-related TEAE |
6.5 |
2.9 |
Hematologic safety profile
- The hematologic safety profile was consistent with the monotherapy profiles of teclistamab and talquetamab.
- The most prevalent hematologic treatment-emergent adverse event (TEAE) was neutropenia (Table 2).
- Febrile neutropenia was observed in 12.9% of patients at all dose levels, and 8.8% of patients at the RP2R dose
- There were no discontinuations due to hematologic AEs.
Table 2. RedirecTT-1 hematologic safety profile
|
RP2R; recommended phase 2 regimen; TEAE, treatment-emergent adverse event. *Adapted from Cohen.1 |
||||
|
TEAE, % |
All dose levels |
RP2R Dose† |
||
|---|---|---|---|---|
|
Any Grade |
Grade 3/4 |
Any Grade |
Grade 3/4 |
|
|
Neutropenia |
65.6 |
61.3 |
55.9 |
44.1 |
|
Anemia |
50.5 |
34.4 |
32.4 |
23.5 |
|
Thrombocytopenia |
43.0 |
29.0 |
32.4 |
23.5 |
Non-hematologic safety profile
- Non-hematological AEs were generally of low grade
- Rates of Grade 3/4 non-hematologic TEAEs were low overall, including at the RP2R
- Five immune effector cell-associated neurotoxicity syndrome events were seen in three patients
- The rate of cytokine release syndrome was 76.3%
- Most cases were Grade 1/2
- 26.9% of patients received tocilizumab
- All cases resolved, with no deaths or discontinuations due to cytokine release syndrome
- Other any Grade AEs of interest were
- dysguesia, reported in 61.3% of patients;
- skin toxicity, reported in 53.8% of patients; and
- nail disorders, reported in 46.2% of patients.
Efficacy
- The overall response rate (ORR) was 86.6% across all dose levels and 96.3% at the RP2R dose (Figure 2).
- Overall ORR was 71.4% in patients with extramedullary disease and 85.7% at the RP2R dose, with >20% of patients achieving ≥complete response.
- The median progression-free survival for the entire cohort was 20.9 months, with a 9-month rate of 77.1% at the RP2R.
- Responses were rapid
- Median time to first response occurred within 2 months
- Median time to best response occurred within 4 months
- Responses were durable, with median response not estimable
- At data cutoff, 61% of patients remained on treatment
Figure 2. Best response at all dose levels and RP2R at median follow-up of 13.4 months and 8.1 months, respectively*
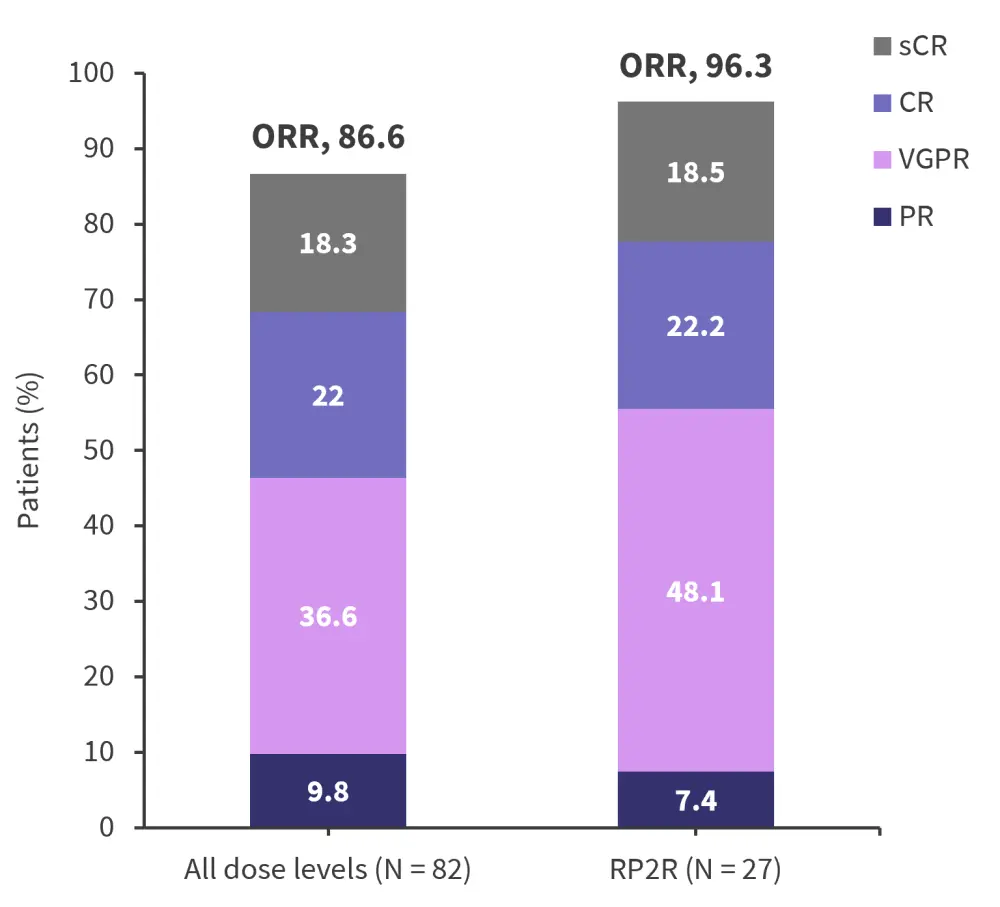
CR, complete response; ORR, overall response rate; PR, partial response; R2PR, recommended phase 2 regimen; sCR stringent complete response; VGPR, very good partial response.
*Adapted from Cohen.1
Conclusion
In this first combination study of a BCMA- and GPRC5D-targeted bispecific antibody, teclistamab plus talquetamab at the RP2R dose demonstrated a clinically manageable safety profile; this was consistent with the respective monotherapy profiles, with low rates of discontinuation and death. These first results from RedirecTT-1 reported an ORR of >96% in patients receiving the RP2R dose and an ORR of >85% in patients with extramedullary disease (a high-risk population with unmet need), supporting further evaluation of the combination.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

