All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Delving into the prospects of mass spectrometry in multiple myeloma
Do you know... Identify one disadvantage from the list below associated with using MALDI-TOF mass spectrometry (MS) for routine monitoring of patients with plasma cell disorders.
Monoclonal proteins (MPs) secreted by plasma cells in patients with multiple myeloma (MM) are used as a biomarker to identify plasma cell disorders and measure disease activity.1 The standard practice to measure MPs includes serum protein electrophoresis and/or serum immunofixation electrophoresis (sIFE). However, these methods are not adequately sensitive to detect low MP concentrations, which is a common feature in patients using highly active therapies. Mass spectrometry (MS) is a technique with greater sensitivity to detect MPs.
The International Myeloma Working Group (IMWG) has recently approved the use of light chain matrix-assisted laser desorption/ionization time-of-flight MS (MALDI-TOF MS), also termed MASS-FIX, instead of sIFE.1 The Multiple Myeloma Hub has previously reported on why MS could substitute IFE in the diagnosis and monitoring of MM, including the advantages of MALDI-TOF MS over IFE.
Recently, several studies have reported on the use MS in patients with MM, including a review published by Giles, et al.1 in the British Journal of Hematology. In this follow-on article, we present a summary of the advantages and disadvantages of MS over other methodologies, such as sIFE and next-generation sequencing (NGS) of bone marrow (BM) samples, in the diagnosis and monitoring of patients with MM, as well as considerations for its future use in clinical practice.
Identification and monitoring of monoclonal proteins1
Two methods developed for the identification of MPs in serum include an intact light chain and a clonotypic peptide approach, which both utilize immune enrichment prior to MS analysis to purify immunoglobulins (Igs). The workflows for both methods are summarized in Figure 1, and the analytical performances of different types of MS assay for MP monitoring are presented in Table 1. The clonotypic peptide method, although complex and time-consuming, offers very high levels of sensitivity (MP detection at <1 mg/L). The liquid chromatography MS (LC-MS) assay and clonotypic peptide methods show high resolution and high specificity, respectively, offering universal elimination of interference with therapeutic monoclonal antibodies.
Figure 1. Workflows for the intact light chain and clonotypic peptide MS assays*
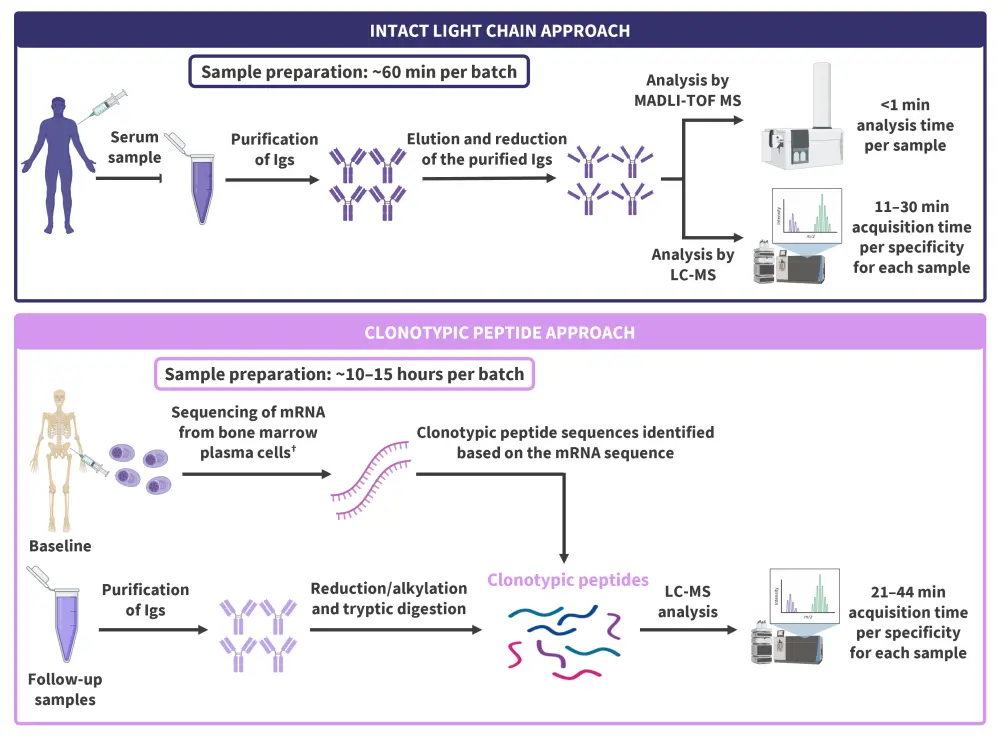
Ig, immunoglobulin; LC, liquid chromatography; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; mRNA, messenger RNA; MS, mass spectrometry.
*Adapted from Giles, et al.1 Created in BioRender.com.
Table 1. Analytical performances of the different types of MS*
|
LC-MS, liquid chromatography-mass spectrometry; LOD, limit of detection; LOQ, limit of quantitation; NR, not reported; MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MS, mass spectrometry; QIP-MS, quantitative immunoprecipitation mass spectrometry. |
||||
|
Citation/s (assay name) |
Analyte |
Type of MS analysis |
LOD, mg/L |
LOQ, mg/L |
|---|---|---|---|---|
|
Mills, et al. 2016 |
Intact light chain |
MALDI-TOF MS |
<100 |
450 |
|
Ashby, et al. 2018 |
Intact light chain |
MALDI-TOF MS |
8–15† |
15† |
|
Mills, et al. 2015 |
Intact light chain |
LC-MS |
5 |
NR |
|
Puig, et al. 2021 |
Intact light chain |
LC-MS |
NR |
NR |
|
Langerhorst, et al. 2021 |
Clonotypic peptide |
LC-MS |
NR |
1 |
|
Liyasova, et al. 2021 |
Clonotypic peptide |
LC-MS |
0.5–1 |
2 |
|
Zajec, et al. 2018 |
Clonotypic peptide |
LC-MS |
0.1–0.3 |
0.4–0.9 |
|
Martins, et al. 2020 |
Clonotypic peptide |
LC-MS |
0.1–1.5 |
NR |
Studies have shown residual MP to be measurable by MALDI-TOF MS in 18–45.2% of patients with complete response (CR). At Day 100 post autologous hematopoietic stem cell transplantation (auto-HSCT), residual MP was also measurable by MALDI-TOF MS in 59% of patients who were in CR and were deemed minimal residual disease (MRD)-negative by next-generation flow (NGF). In a study by Abeykoon, et al.2, MPs were detected to a greater extent in IgG myeloma (87%) compared with IgA and free light chain (FLC) myeloma (33% and 30%, respectively).
For patients treated in the STAMINA trial (NCT01109004), the prognostic values of MALDI-TOF MS positivity and MRD positivity were retained at 1-year post-randomization, whereas this was not the case for sIFE positivity. However, MALDI-TOF MS showed variable sensitivity in the detection of low-level monoclonal FLC when compared to the Freelite assay (a specific test to measure kappa and lambda FLCs developed by The Binding Site). This warrants the use of serum FLC and Bence Jones protein assessments along with MALDI-TOF MS assays that do not include reagents designed to detect monoclonal FLC.
Using MALDI-TOF MS, low levels of MPs were measurable in 91% of samples (Giles, et al.3) and in 100% of patients (GEMMENOS65 trial; NCT01916252) with non-secretory MM, allowing serological monitoring and, thus, future possibilities for these patients to be better monitored and enrolled in clinical trials.
Although LC-MS offers greater sensitivity than MALDI-TOF MS assays, it is more time-intensive. Quantitative immunoprecipitation mass spectrometry (QIP-MS), LC-MS, and NGS MRD assessments have shown consistency, with residual MPs measurable by LC-MS in 100% of samples in a study by Derman, et al.4 Although fewer clinical studies have assessed the clinical utility of the clonotypic peptide approach, the results from some preliminary studies are encouraging. A study by Bergen, et al.5 showed that residual clonotypic peptide was measurable in the serum of 91% of patients in CR, including 77% of patients who were MRD negative by six-color flow cytometry. In another study using EasyM (a non-invasive, sensitive, MS-based assay), residual clonotypic peptide was detectable in 89% of samples, including 86% of MRD-negative samples (Liyasova, et al.6). Good agreement has also been reported between NGS and clonotypic peptide MRD assessments; concordance was observed between NGS and MS in 79% of samples and the longest progression-free survival was observed in patients who were negative by both NGS and MS.
Diagnostic performance of MS assays in patients with light chain amyloidosis1
A study from the UK National Amyloidosis Centre showed that FLC MALDI-TOF MS had high sensitivity in detecting monoclonal FLC; FLC was measurable in 100% of baseline serum samples by FLC MALDI-TOF MS, compared with in 48%, 74%, and 91% of samples by serum protein electrophoresis, sIFE, and Freelite, respectively. In addition, FLC MALDI-TOF MS identified residual monoclonal FLC in 76% of patients who achieved hematologic CR during a 1-year follow-up. Furthermore, MALDI-TOF MS-positive patients had a shorter time to progression compared with those who achieved MALDI-TOF MS negativity (75% vs 13%; p = 0.003). MALDI-TOF MS and LC-MS-based assays also appear to detect N-linked glycosylation of monoclonal light chain, enabling early diagnosis since its prevalence is particularly high in patients with kappa light chain-associated light chain amyloidosis.
Future considerations for MS1
The advantages and disadvantages of using current IMWG-approved methodologies are outlined in Table 2. There are several challenges related to the use of MS assays, and future research is needed to address them. BM-based MRD assessment using NGS and/or NGF remains the gold standard for MRD monitoring due to its greater sensitivity; however, its testing frequency is limited by invasive BM sampling and a high cost. A subanalysis from the GEM2012MENOS65 trial has shown a high negative predictive value (NPV) of MALDI-TOF MS post-consolidation compared with NGF; the NPV of MALDI-TOF MS post-consolidation was 97%, 89%, and 85% per the MRD ranges of ≥1 × 10−4, ≥1 × 10−5 to 1 × 10−4, and ≥1 × 10−6, respectively. Further studies are required to evaluate if MALDI‑TOF MS may offer a peripheral blood-based alternative in patients being evaluated for MRD to reduce the frequency of BM-based assessments.
According to some cost-effectiveness models, the MRD-guided discontinuation of post-HSCT therapy may be cost-effective due to the reduced costs of drugs, but its impact on patient outcomes is an active field of research, with several ongoing clinical trials investigating this.
Due to the high sensitivity of MS, detection of oligoclonal peaks is a common feature of myeloma in patients undergoing treatment even prior to auto-HSCT; therefore it’s important to analyze baseline samples to ensure transient oligoclonal peaks can be distinguished from disease related-MPs. Furthermore, MS assays can identify a small proportion of patients with IgG kappa myeloma, provided that a complete clinical history of the patient is available.
The standardized response assessments might need to be redefined by the IMWG if MS assays were to be adopted on a large scale. Patients will also need to be appropriately counseled about the greater sensitivity and higher rates of residual MP. Asymptomatic individuals should not be screened using MS assays outside a clinical trial setting.
Table 2. Advantages and disadvantages of methodologies approved by the IMWG*
|
FLC, free light chain; IMWG, International Myeloma Working Group; MALDI-TOF MS, matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry; MM, multiple myeloma; MP, monoclonal protein; MRD, minimal residual disease; NGF, next-generation flow; NGS, next-generation sequencing; PCD, plasma cell disorders; PET-CT, positron-emission tomography-computed tomography; sIFE, serum immunofixation electrophoresis; SPE, serum protein electrophoresis; tmAb, therapeutic monoclonal antibody. |
|||
|
Methodology |
Cost, $ |
Advantages |
Disadvantages |
|---|---|---|---|
|
SPE |
7–67 |
Widely available |
Interference from tmAbs |
|
sIFE |
22–200 |
Widely available |
Interference from tmAbs |
|
Serum FLC |
34–120 |
Widely available |
Does not specifically measure the monoclonal FLC and relies on the FLC ratio to detect monoclonal FLC, making it more difficult to interpret in the context of renal impairment due to the reduced clearance rate |
|
Intact light chain MALDI-TOF MS |
145 |
Greater sensitivity |
Only available in the USA |
|
NGF |
450 |
High sensitivity |
Requires BM biopsy to obtain a suitable sample |
|
NGS |
1,950 |
High sensitivity |
Requires BM biopsy to obtain a suitable sample |
|
PET-CT |
870 |
Can monitor intra-and extra-medullary disease |
False-negative results were observed in patients with hexokinase deficiency |
Conclusion
MS assays can measure residual monoclonal FLC within a polyclonal background, providing greater sensitivity. The sensitivity of gel-based methods can be enhanced by using antisera specific for serum FLC. MS assays are also particularly helpful in patients with renal impairment who, due to higher levels of polyclonal FLC background, may require higher levels of monoclonal FLC production to skew the ratio outside of the renal reference range. MS assays can play an important role in identifying and distinguishing the monoclonal FLC from the polyclonal background and addressing the underlying cause, including refining the diagnostic criteria for FLC monoclonal gammopathy of undetermined significance.
MS assays may have the potential to be the new diagnostic and monitoring method of choice for MPs in serum due to their higher throughput capabilities and low cost. Of the various MS assays, MALDI-TOF MS assays are the most suitable alternative to the current electrophoretic techniques. However, the need for the investment in specialized equipment and uncertainty around reagent costs may hinder the use of MALDI-TOF MS. Further research is warranted to establish the complementary role of MS assays in MRD monitoring and compare MS assays against peripheral blood-based techniques. On the other hand, LC-MS assays are not likely to be suitable for large-scale routine use in clinical practice due to longer processing times and a lack of automated sample processing. Future studies should be conducted in collaboration to align the different MS and other existing methodologies for assessing disease response.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




