All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
CD43 as an adverse prognostic marker for MM and other hematologic malignancies
Do you know... Which of the following is not associated with increased CD43 expression at MM diagnosis?
Extensive efforts have been put into the design, development, and revision of multiple myeloma (MM) staging criteria to include molecular genetic risk factors alongside clinical parameters.1 However, widespread adoption of these staging criteria has been challenged by accessibility to technology and consistency of application.2
Furthermore, considerable research is being conducted to identify other biological markers that can be incorporated into existing criteria to increase their sensitivity and specificity for prognostic and risk stratification, thereby guiding treatment choices, especially in first- and second-line treatment.
CD43, also known as sialophorin, is expressed on the cell surface of bone marrow hematopoietic stem cells, and it has roles in cell cycle control, cell growth and differentiation, and cell survival/apoptosis.2 Overexpression of CD34 has been identified in other hematologic malignancies, including acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndromes, chronic lymphocytic leukemia. In addition, CD43 overexpression in a non-germinal center B-cell subgroup of patients with diffuse large B-cell lymphoma has been associated with poorer outcomes. Therefore, CD43 may be a promising therapeutic target for antibody-mediated therapies.2
Here, the Multiple Myeloma Hub considers the work of Xueqin Ning and colleagues.2 Reported in Leukaemia and Lymphoma in June 2022, we summarize the data from their observational, biological study exploring the potential of CD43 as a marker of disease risk, treatment response, and clinical outcomes in patients with newly diagnosed MM (NDMM).
Study design
The study was a single-center, prospective, biological observation study conducted between January 2017 and December 2019. Median follow-up was 22 months (range, 1–44 months).
Patients described as CD43-positive were defined as those identified with monoclonal plasma cells expressing CD43, as detected by multiparameter flow cytometry.
Data collection included:
- Demographic data
- Diagnostic and clinical data
- Biological data via bone marrow aspiration
- Flow cytometry data to assess antigen expression on all cells expressing CD38 and CD138
- Treatment response and clinical outcome data (for ≥12 months)
Treatment
Patients received four cycles of one of the following induction therapies:
- Bortezomib, cyclophosphamide, and dexamethasone (VCd)
- Bortezomib, lenalidomide, and dexamethasone (VRd)
- Bortezomib, doxorubicin, and dexamethasone (PAD)
- Bortezomib, thalidomide, and dexamethasone (VTd)
- Bortezomib and dexamethasone (Vd)
The induction therapy was followed by further chemotherapy or autologous stem cell transplant. Treatment response was assessed according to International Myeloma Working Group (IMWG) 2016 efficacy criteria (which we’ve previously covered here).
Results
In total, 109 patients were recruited and ~25% were aged >65 years. Furthermore, 70.6% of patients were CD34-positive, and 98 patients (~90%) had chromosomal abnormalities. Further baseline patient characteristics can be seen in Table 1.
Table 1. Demographic and base line characteristics of patients*
|
*Adapted from Ning, et al.2 |
||||
|
Parameter, n % |
All patients |
CD43-positive patients |
CD43-negative patients |
p value |
|---|---|---|---|---|
|
Age |
|
|
|
|
|
>65 years |
29 (25.6) |
19 (24.7) |
10 (31.2) |
0.479 |
|
≤65 years |
80 (73.4) |
58 (75.3) |
22 (68.6) |
|
|
Male |
68 (62.4) |
46 (59.7) |
22 (68.8) |
0.377 |
|
Female |
41 (37.6) |
31 (40.3) |
10 (31.3) |
|
|
FISH |
98 |
70 |
28 |
|
|
del(13q14) |
25 (25.5) |
22 (31.3) |
3 (10.7) |
0.034 |
|
del(17p) |
9 (9.2) |
8 (11.4) |
1 (3.6) |
0.407 |
|
IGH translocation |
37 (37.8) |
30 (42.9) |
7 (25.0) |
0.099 |
|
Unknown |
11 |
7 |
4 |
|
Overall, almost three-quarters of patients (72.5%) received bortezomib-based treatment, with 58.7% receiving VCd. Approximately one-third of patients underwent ASCT after induction with 73 patients receiving maintenance chemotherapy.
Patients with CD43 expression, compared with those without, were more likely to present with:
- International Staging System (ISS) Stage III (67.5% vs 46.9%; p = 0.044)
- Anemia (64.9% vs 37.5%; p = 0.008)
- A higher percentage of monoclonal plasma cells on multi-flow cytometry (5.5% vs 1.4%; p = 0.003)
After four cycles of chemotherapy, CD43-positive patients were more likely to exhibit a worse treatment response compared with CD43-negative patients (p = 0.021), as well as a lower rate of very good partial remission or better (35.1% vs 56.3%; p = 0.041; Figure 1). A lower overall response rate was also seen for CD43-postive patients (75.3% vs 84.4%; p = 0.299)
Figure 1. Treatment response according to presence of CD43 expression*
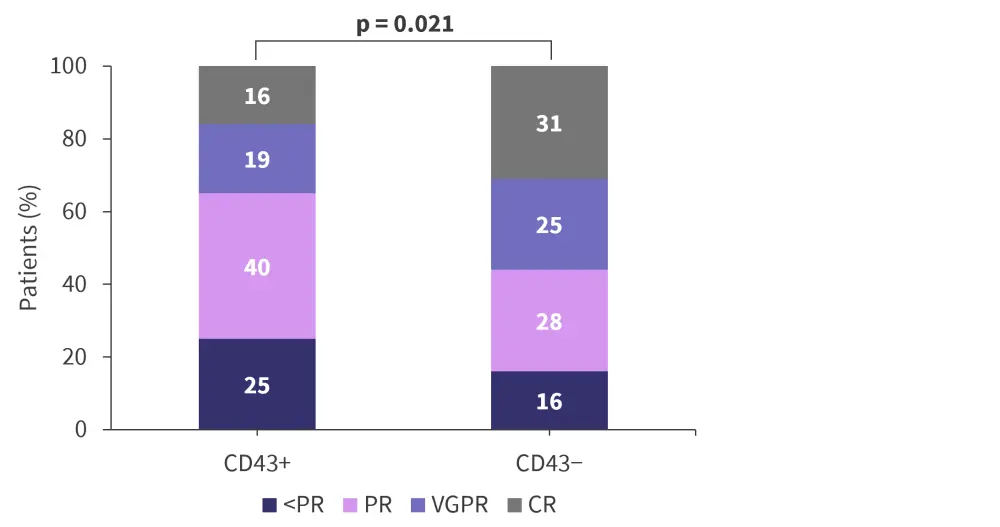
CR, complete remission; PR, partial remission; VGPR, very good partial remission.
*Adapted from Ning, et al.2
CD43-positivity was identified as an independent prognostic marker in terms of both overall survival and progression-free survival on both univariate and multivariate analysis (Table 3).
Table 3. Effects of CD43-positivity on PFS and OFS as an independent variable*
|
CI, confidence interval; OS, overall survival; PFS, progression-free survival. |
|
|
Analysis |
Hazard ratio for CD43-positivity (95% CI; p value) |
|---|---|
|
PFS univariate |
2.517 (1.178–5.376; p = 0.017) |
|
PFS multivariate |
3.148 (1.421–6.975; p = 0.005) |
|
OS univariate |
3.664 (1.100–12.075; p = 0.034) |
|
OS multivariate |
4.939 (1.395–17.488; p = 0.013) |
Conclusion
Overall, Ning, et al. demonstrated that CD43-positive patients with NDMM are more likely to present with an advanced ISS stage, anemia, 13q deletion, and a higher percentage of monoclonal plasma cells. More significantly, CD43 expression was identified as an independent prognostic factor for MM, with potential utility as a predictive marker of poorer treatment response and clinical outcome. CD43 needs to be investigated in larger clinical trials as a potential marker for risk stratification, prognostication, and treatment selection.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




