All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
CARTITUDE-2 Cohort C: Efficacy and safety of cilta-cel in patients with relapsed/refractory MM
Several therapies targeting B-cell maturation antigen (BCMA) are now available for the treatment of patients with multiple myeloma (MM); therefore, understanding the sequencing of these therapies is important. Ciltacabtagene autoleucel (cilta-cel) has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with relapsed/refractory (R/R) MM.
During the 49th Annual Meeting of the EBMT, María-Victoria Mateos, co-chair of the Multiple Myeloma Hub Steering Committee, presented data from Cohort C of the CARTITUDE-2 trial evaluating the efficacy and safety of cilta-cel in patients with progressive MM exposed to non‑cellular anti‑BCMA immunotherapy.1 The Multiple Myeloma Hub has previously reported results from CARTITUDE-1 and Cohort B of the CARTITUDE-2 trial. We are pleased to present key results from Cohort C of the CARTITUDE-2 trial here.
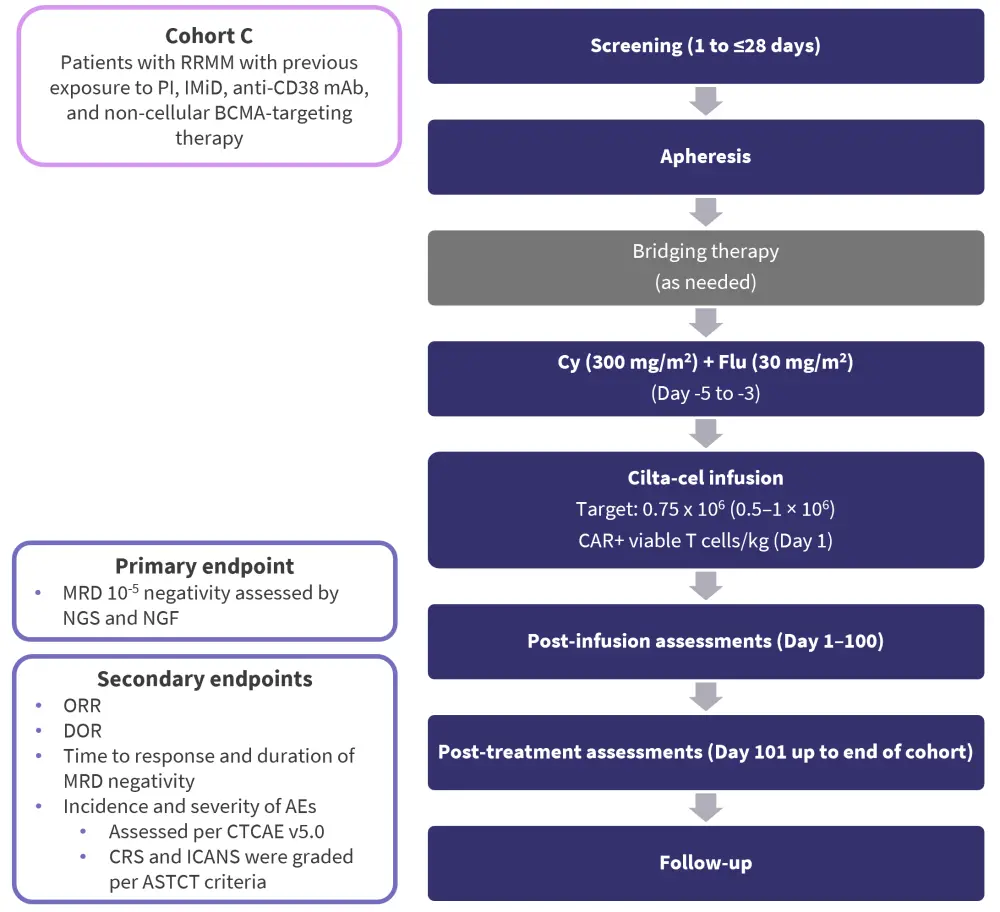
Study design1
The CARTITUDE-2 trial (NCT04133636) is a phase II, multi-cohort study of cilta-cel in patients with R/R MM. The overall study design and the primary and secondary endpoints of CARTITUDE-2 Cohort C are shown in Figure 1.
Figure 1. Study design and endpoints*
AE, adverse event; ASTCT, American Society for Transplantation and Cellular Therapy; BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; cilta-cel; ciltacabtagene autoleucel; CRS, cytokine release syndrome; CTCAE, Common Terminology Criteria for AEs, Cy, cyclophosphamide; DOR, duration of response; Flu, fludarabine; ICANS, immune effector cell-associated neurotoxicity syndrome; IMiD, immunomodulatory drug; mAb, monoclonal antibody; MRD, minimal residual disease; NGF, next-generation flow; NGS, next-generation sequencing; ORR, overall response rate; PI, protease inhibitor; RRMM, relapsed/refractory multiple myeloma.
*Adapted from Mateos.1
Results1,2
Baseline characteristics
A total of 20 patients were included in Cohort C, of which 65% were exposed to an antibody–drug conjugate (ADC) and 35% to a bispecific antibody (BsAb). Key baseline characteristics for these patients are shown in Table 1.
The median time from last anti-BCMA treatment to cilta-cel infusion was 6.4 months (range, 2.0–24.6 months).
Table 1. Baseline characteristics
|
ADC, antibody–drug conjugate; BCMA; B-cell maturation antigen; BM, bone marrow; BsAb, bispecific antibody; CR, complete response; IMiD; immunomodulatory drugs ISS, International Staging System; LOT, line of therapy; MM, multiple myeloma; PD, progressive disease; PI, protease inhibitor; sCR, stringent CR; SD, stable disease; VGPR, very good partial response. |
|||
|
Characteristic, % (unless otherwise stated) |
Total Cohort C |
ADC-exposed cohort |
BsAb-exposed cohort |
|---|---|---|---|
|
Median age (range), years |
62.5 (44–81) |
66.0 (44–81) |
60.0 (49–71) |
|
Male |
60 |
61.5 |
57.1 |
|
Race |
|
|
|
|
White |
95 |
100 |
85.7 |
|
Black |
5 |
0 |
14.3 |
|
BM plasma cells ≥60%‡ |
32 |
33.3 |
28.6 |
|
Extramedullary plasmacytomas |
25 |
38.5 |
0 |
|
High-risk cytogenetic profile§ |
15 |
15.4 |
14.3 |
|
Median time from initial MM diagnosis (range), years |
6.3 (2.5–16.3) |
6.4 (3.6–16.3) |
5.0 (2.5–14.5) |
|
Disease stage, ISS |
|
|
|
|
I |
40 |
46.2 |
28.6 |
|
II |
20 |
23.1 |
14.3 |
|
III |
40 |
30.8 |
57.1 |
|
Median number of prior LOTs, (range) |
8 (4–13) |
8 (4–13) |
8 (6–12) |
|
Last line of therapy |
|
|
|
|
Anti-BCMA |
30 |
30.8 |
28.6 |
|
Other treatments |
70 |
69.2 |
71.4 |
|
Response to prior anti-BCMA* |
|
|
|
|
sCR |
5 |
7.7 |
0 |
|
CR |
5 |
0 |
14.3 |
|
VGPR |
15 |
15.4 |
14.3 |
|
SD/PD |
75 |
— |
— |
|
Refractory status |
|
|
|
|
Triple class‖ |
90 |
84.6 |
100 |
|
Penta-drugs¶ |
55 |
53.8 |
57.1 |
|
Anti-BCMA treatment |
90 |
84.6 |
71.4 |
|
To last LOT |
95 |
100 |
85.7 |
Efficacy
The data cutoff was June 2022.
- At a median follow-up of 18 months, seven of ten patients were minimal residual disease (MRD)-negative; five in the ADC-exposed cohort and two in the BsAb-exposed cohort.
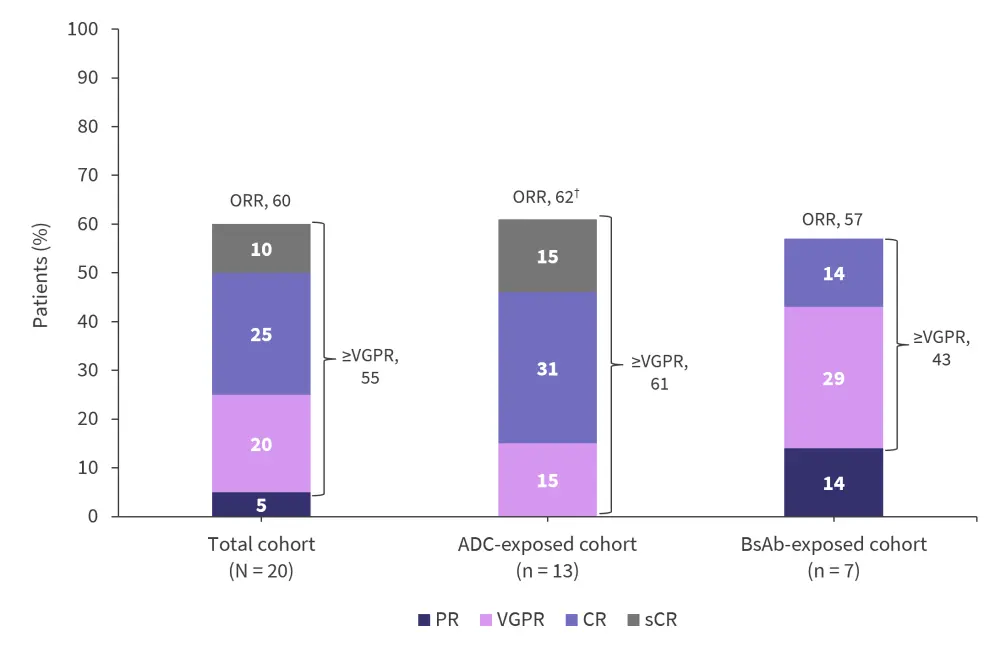
- The median duration of response was 12.3 months, with an overall response rate (ORR) of 60% in the total cohort.
- ORR was similar in both the ADC- and BsAb-exposed cohorts (Figure 2).
- Median progression-free survival was:
- 9.1 months (range, 1.5–13.2 months) in the total cohort
- 9.5 months (range, 1.0–15.2 months) in the ADC-exposed cohort
- 5.3 months (range, 0.6 months to not evaluable) in the BsAb-exposed cohort
- Patients responding to cilta-cel showed a shorter median duration of last anti-BCMA exposure versus those not responding to cilta-cel (29.5 days vs 63.5 days).
- Cilta-cel responders showed a longer time from last anti-BCMA treatment to apheresis versus non-cilta-cel responders (161 days vs 56.5 days).
Figure 2. Response rates*
ADC, antibody–drug conjugate; BsAb, bispecific antibody; CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent CR; VGPR; very good partial response.
*Adapted from Mateos.1
†Percentages may not sum appropriately due to rounding.
Safety
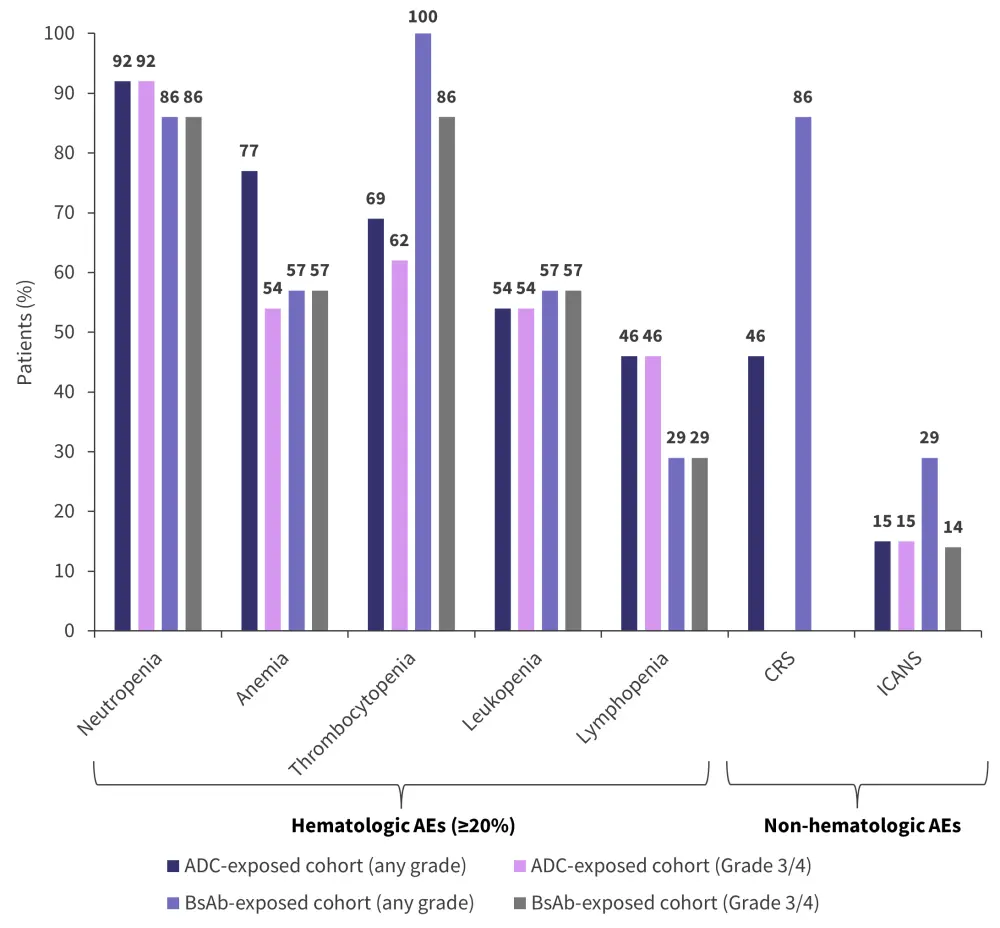
- As shown in Figure 3, the most common adverse events (AEs) were hematologic events.
- Cytokine release syndrome (CRS) of Grade 1 or 2 was observed in 12 of 20 patients, with a median time to CRS onset of 7.5 days (range, 2–10 days).
- Any grade immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in four patients, while Grade 3 or 4 ICANS occurred in three patients.
- The median time to ICANS onset was 9 days (range, 4–13 days) and the median duration was 7 days (range, 4–20 days).
- CRS resolved in all patients, while ICANS resolved in three out of four patients.
- There were no cases of movement and treatment-emergent AEs or parkinsonism reported.
- A total of 12 deaths were reported, due to the following:
- Progressive disease (n = 8)
- COVID-19 pneumonia (n = 2)
- Clostridiodes difficile colitis (n = 1; treatment-related)
- Subarachnoid hemorrhage (n = 1)
Figure 3. Adverse events*
ADC, antibody–drug conjugate; AE, adverse event; BsAb, bispecific antibody; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome.
*Data from Mateos.1
Conclusion
Findings from Cohort C of the CARTITUDE-2 trial demonstrated a clinical benefit of cilta-cel in patients with R/R MM who were heavily pretreated and exposed to non‑cellular anti‑BCMA immunotherapy. The study highlighted the impact of prior anti‑BCMA therapy on responses to cilta‑cel. The safety profile of cilta‑cel was consistent with that observed in the CARTITUDE-1 trial. The data from Cohort C of the CARTITUDE-2 trial may inform future treatment plans, including sequencing and a washout period between BCMA‑targeted agents.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



