All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
CARTITUDE-2 Cohort B: Results of cilta-cel in patients with early relapsed MM
Patients with multiple myeloma (MM) who experience an early relapse have a poor prognosis, with a median survival of <2 years. This represents a clear unmet need for this group despite the use of proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and monoclonal antibodies.1
Ciltacabtagene autoleucel (cilta-cel) has recently been approved in the US and Europe for patients with relapsed/refractory MM based on the CARTITUDE-1 study. After more than 2 years of median follow-up, this phase Ib/II trial reported durable and deepening responses with cilta-cel, with 83% of patients achieving a stringent complete response that led to 27-month progression-free survival and overall survival rates of 54.9% and 70.4%, respectively.2
During the European Hematology Association (EHA)2022 Congress, Niels Van de Donk3 presented an update of the CARTITUDE-2 trial of cilta-cel in patients with MM who experience early relapse. This update covered data from cohort B, which included patients experiencing early relapse following initial treatment with a PI and IMiD (early relapse defined as progression within 12 months of stem cell transplant or within 12 months from start of anti-MM therapy for patients who have not received stem cell transplant).1
Study design1
The endpoints for this study and the overall study design are shown in Figure 1. Patients were given one infusion of cilta-cel 4−6 days after lymphodepletion therapy with fludarabine and cyclophosphamide.
Figure 1. Endpoints and study design*
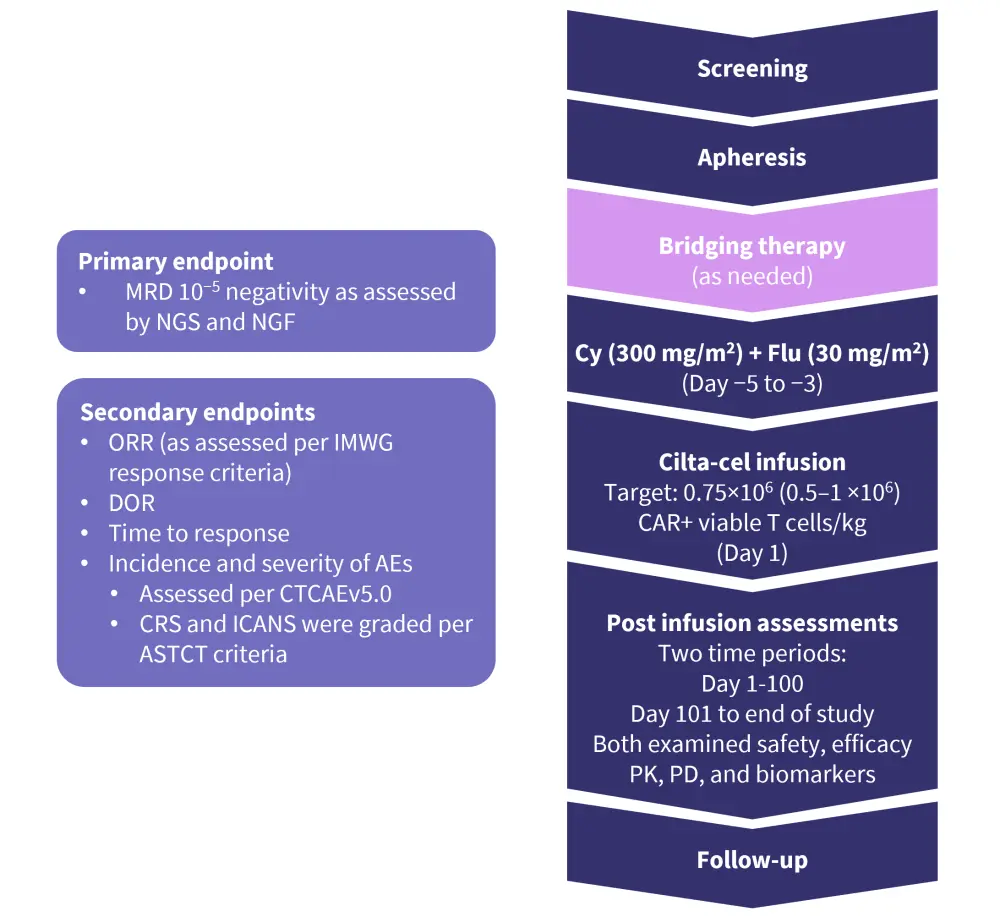
AE, adverse event; ASTCT, American Society for Transplantation and Cellular Therapy; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; CTCAE, common terminology criteria for adverse events; Cy, cyclophosphamide; DoR, duration of response; Flu, fludarabine; ICANS, immune effector cell-associated neurotoxicity; IMWG, international Myeloma Working Group; MRD, measurable residual disease; NGF, next-generation flow; NGS, next-generation sequencing; ORR, overall response rate; PD, pharmacodynamics; PK, pharmacokinetics.
*Adapted from Agha, et al.1
Results1
Patient characteristics
With a median follow-up of 13.4 months (range, 5.2−21.7 months), 19 patients received cilta-cel, with the last data cut-off in January 2022. Patient characteristics at baseline are shown in Table 1. The median age of patients in this trial was 58 years and 73.7% were male. This cohort included 78.9% of patients who were refractory to last treatment and 15.8% who were triple-class refractory. In addition, 78.9% of patients had received a previous autologous stem cell transplant and one patient was treated in an outpatient setting.
Table 1. Baseline patient characteristics*
|
LOT, line of therapy; SCT, stem cell transplant. |
|
|
Characteristics, % (unless otherwise stated) |
Total (N = 19) |
|---|---|
|
Median age (range), years |
58 (44−67) |
|
Male |
73.7 |
|
Black/African American |
10.5 |
|
Extramedullary plasmacytomas |
15.8 |
|
Bone marrow plasma cells ≥60% |
21.1 |
|
High-risk cytogenetic profile |
15.8 |
|
Del17p |
15.8 |
|
Median prior LOT (range) |
1 (1−1) |
|
Previous autologous SCT |
78.9 |
|
Triple-class exposed |
21.1 |
|
Triple-class refractory |
15.8 |
|
Refractory status |
|
|
Lenalidomide |
78.9 |
|
Bortezomib |
31.6 |
|
Daratumumab |
15.8 |
|
Thalidomide |
10.5 |
|
Refractory to last line of therapy |
78.9 |
|
Median duration between diagnosis and enrollment (range), years |
1.15 (0.5−1.9) |
Efficacy
In this population, an overall response rate (ORR) of 100% was achieved (95% confidence interval [CI], 82.4–100; Figure 2). The median time to first response was 1.0 month (range, 0.9−9.7 months), while the median time to best response was 5.1 months (range, 0.9−11.8 months). The median duration of response was not reached as of the current data cut-off.
The 12-month progression-free survival rate was 89.5% (95% CI, 64.1−97.3). Out of the whole cohort, 15 were evaluable for measurable residual disease (MRD) assessment and 93.3% of these were MRD negative (95% CI, 68.1−99.8) at a threshold of 10−5.
Figure 2. Response rates*
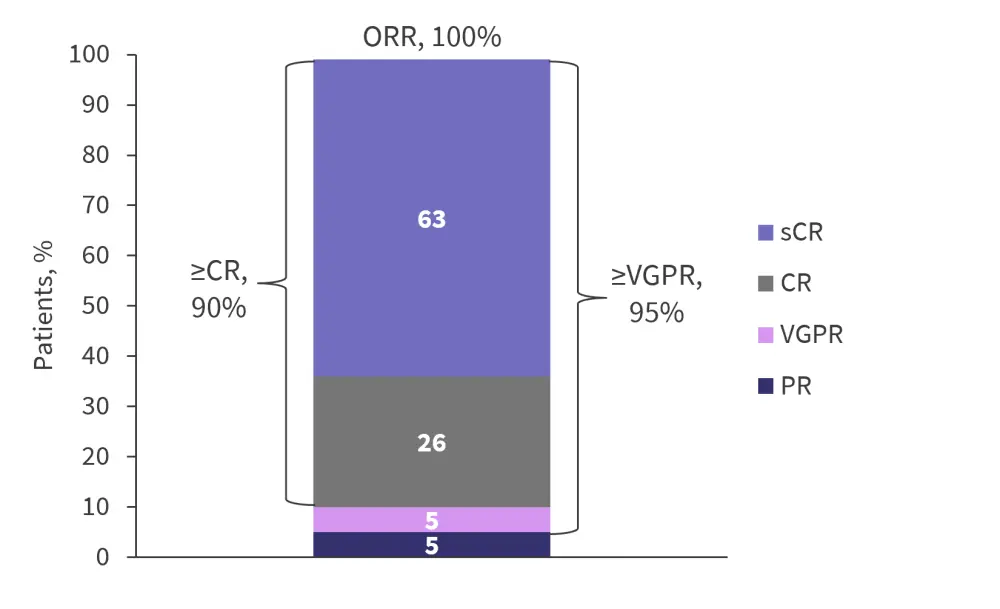
CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Agha, et al.1
Safety
Neutropenia occurred in 95% of patients, with 90% experiencing Grade 3 or 4. Of the patients experiencing Grade 3 or 4 cytopenias, 16% of cases of thrombocytopenia and 11% of lymphopenia and neutropenia had not recovered to Grade 2 by Day 60. Cytokine release syndrome (CRS) occurred in 84% of patients, with one patient having a Grade 3/4 event (Figure 3). The median time to CRS onset was 8 days (range, 5−11 days) and each event lasted for a median of 3.5 days (range, 1−7 days). Resolution of CRS occurred in all patients, with 63% receiving tocilizumab.
Only one patient experienced Grade 1 immune effector cell-associated neurotoxicity (ICANS) 11 days after infusion, which lasted for 4 days. The sponsor implemented patient management strategies across the cilta-cel clinical trials to reduce the initial incidence of movement and neurocognitive treatment-emergent adverse events reported in the CARTITUDE-1 trial.
Figure 3. Adverse events*
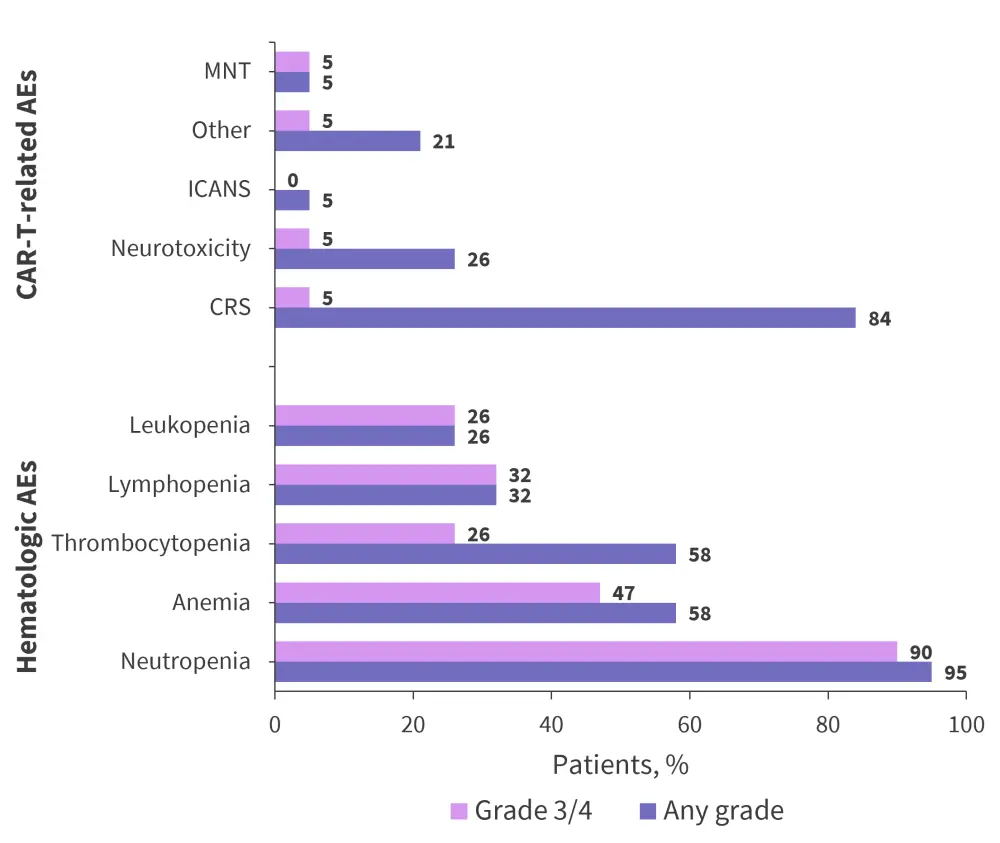
AEs, adverse events; CRS, cytokine release syndrome; ICANS immune effector cell-associated neurotoxicity; MNT, movement and neurocognitive treatment-related adverse event.
*Adapted from Agha, et al.1
One case of a Grade 3 movement and neurocognitive event was recorded. This patient was treated with high-dose methylprednisolone, plasmapheresis, and intravenous immunoglobulin, and was stable at the last data cut-off with a complete response to cilta-cel.4
There was one death recorded on the trial due to progressive disease at Day 158 following cilta-cel infusion.
Conclusion
In patients with MM who experienced early clinical relapse after initial treatment, one infusion of cilta-cel produced promising results. An ORR of 100% was achieved, with 90% of patients reaching at least a complete response. The safety profile was deemed to be manageable with mostly low-grade CRS, and the majority of cytopenias resolved to Grade 2 by Day 60. Cilta-cel represents a good option for the treatment of these higher-risk patients who experienced early relapse and supports continued investigation of the use of cilta-cel in earlier lines of treatment.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


