All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
The IMWG Revised International Staging System for multiple myeloma: Proposed second revision
Do you know... In the second revision of the International Staging System (R2-ISS) for MM, which of the following is allocated 0.5 points if it is identified in a patient?
The Multiple Myeloma Hub has previously covered the identification and management of patients with multiple myeloma (MM) with high-risk disease, including the use of the International Myeloma Working Group (IWMG) Revised International Staging System (R-ISS). The R-ISS guidelines are considered the standard risk stratification model for patients with newly diagnosed MM (NDMM) and have been widely adopted into clinical practice and research.1,2
A significant limitation of the R-ISS guidelines is that nearly two-thirds of patients (62%) are classified as intermediate-risk (R-ISS II), possibly including patients with lower or increased risk of disease progression or risk. Further to this, since the development of R-ISS, other independent factors indicating poor prognosis NDMM have been identified, including 1q gain or amplification, and potentially the negative compound effect of cytogenetic abnormalities, such as del(17p), when present together.
The European Union has funded an international project called the HARMONY project,1 through which the European Myeloma Network (EMN) has conducted a large cohort analysis of patients with NDMM, to explore the integration of extra risk features into the R-ISS. Published in the Journal of Clinical Oncology in May 2022, D’Agostino et al.2 present their proposed second revision of the R-ISS guidelines (R2-ISS) and investigate the potential impact of the classification of MM. In addition, Chen et al.3 performed a real-world analysis of the clinical relevance of R2-ISS for patients undergoing induction treatment. The Multiple Myeloma Hub is pleased to provide a summary of these publications here.
HARMONY R2-ISS2
Study design
Data were collected from 10,843 patients with NDMM who were enrolled in 16 clinical trials across Europe. The study explored prognostic markers, including ISS disease stage, presence of del(17p) and t(4:14), lactate dehydrogenase levels, and 1q1 amplification/deletion, to define the R2-ISS criteria. For the investigation set, 2,226 patients out of 7,072 had all variables present and were included in multivariate analysis, with 1,214 similar patients out of 3,771 included in a subsequent replication set.
Results
The median follow-up of patients was 75 months and the clinical variables of patients in the analysis (training) and validation sets are presented in Table 1.
Table 1. Clinical variables of patients in the analysis (training) and validation sets*
|
1q1, 1q gain/amplification; ASCT, autologous stem-cell transplantation; del, deletion; IMiD, immunomodulatory drug; IQR, interquartile range; ISS, International Staging System; LDH, lactate dehydrogenase; NTE, non-transplant-eligible; PI, proteasome inhibitor; t, translocation; ULN, upper limit of normal. |
||||
|
Characteristic, % (unless stated otherwise) |
Training set |
Validation set |
||
|---|---|---|---|---|
|
Total population |
R2-ISS score calculation |
Total population |
R2-ISS score calculation |
|
|
Median age, (IQR), years |
62 (55─70) |
60 (54─65) |
68 (60─74) |
68 (60.25─74) |
|
Age >65 years |
38 |
23 |
58 |
59 |
|
Sex |
|
|
|
|
|
Female |
45 |
43 |
42 |
40 |
|
Male |
55 |
57 |
58 |
60 |
|
ISS |
|
|
|
|
|
I |
36 |
37 |
26 |
23 |
|
II |
40 |
38 |
42 |
46 |
|
III |
25 |
25 |
32 |
32 |
|
LDH |
|
|
|
|
|
>ULN |
14 |
16 |
32 |
31 |
|
del(17p) present |
1 |
12 |
9 |
9 |
|
t(4;14) present |
13 |
12 |
11 |
11 |
|
1q1 present |
36 |
37 |
34 |
33 |
|
Treatment |
|
|
|
|
|
IMiDs |
40 |
23 |
89 |
87 |
|
IMiDs/PIs |
46 |
67 |
11 |
13 |
|
PIs |
15 |
11 |
— |
— |
|
ASCT eligibility |
|
|
|
|
|
NTE |
35 |
17 |
47 |
47 |
Multivariate analysis confirmed ISS, del(17p), lactate dehydrogenase, t(4;14), and 1q anomalies had the highest impact on progression-free survival (PFS) and overall survival (OS). Statistical analysis was used to calculate the relative effect of each risk on OS and PFS, and allocate points according to impact (Table 2).
Table 2. Relative impact of risk factors on OS and PFS and classification points*
|
1q1, 1q gain/duplication; CI, confidence interval; del, deletion; HR, hazard ratio; ISS, international staging system; LDH lactate dehydrogenase; OS, overall survival; PFS, progression-free survival; t, translocation. |
|||
|
Risk feature |
OS, HR (95% CI) |
PFS, HR (95% CI) |
Score value |
|---|---|---|---|
|
1q1 |
1.47 (1.29─1.68) |
1.33 (1.20─1.48) |
0.5 |
|
ISS II |
1.75 (1.49─2.05) |
1.43 (1.28─1.61) |
1 |
|
ISS III |
2.53 (2.13─3.01) |
1.76 (1.54─2.01) |
1.5 |
|
del(17p) |
1.82 (1.53─2.17) |
1.43 (1.23─1.65) |
1 |
|
Elevated LDH |
1.60 (1.36─1.88) |
1.37 (1.20─1.57) |
1 |
|
t(4;14) |
1.53 (1.29─1.81) |
1.40 (1.21─1.62) |
1 |
Using the aggregate scores of the four risk factors, an overall four-tier risk stratification was designed, with two intermediate risk levels (low-intermediate and intermediate-high):
- Low risk (R2-ISS-I, 19.2%; 0 points)
- Low-intermediate risk (R2-ISS-II, 30.8%; 0.5-1 points)
- Intermediate-high risk (R2-ISS-III, 41.2%; 1.5-2.5 points)
- High risk (R2-ISS-IV, 8.8%; 3-5 points)
Median OS and PFS according to R2-ISS can be seen in Figure 1.
Figure 1. Median OS and PFS according to R2-ISS stage*
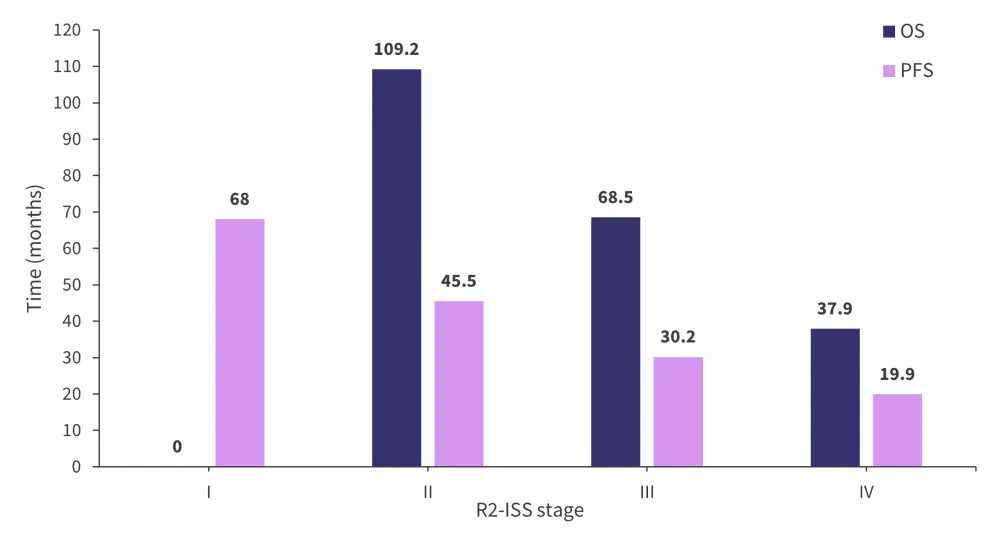
OS, overall survival; PFS, progression-free survival; R2-ISS, second revision of the International Staging System.
*Adapted from D’Agostino, et al.2
A statistical difference (p < 0.001) was identified in the median OS of R-ISS II patients reclassified in the training and validation cohorts, with respective figures of 111 months vs 89 months for patients with R2-ISS II, 71 months vs 56 months for patients with R2-ISS III, and 57 months vs 27 months in patients with R2-ISS IV. D’Agostino et al. conclude that this identifies patients with R-ISS II as highly heterogeneous, and that the R2-ISS criteria can more accurately discriminate between levels of risk.
R2-ISS and real-world implications3
Chen et al. conducted a retrospective analysis of 474 patients with NDMM treated with immunomodulatory drugs or proteosome inhibitors at a single treatment center between January 2010 and January 2022.3 Demographic and patient data can be seen in Table 3.
Table 3. Clinical characteristics of real-world R2-ISS study participants*
|
1q1, 1q gain/amplification; ASCT, autologous stem cell transplantation; del, deletion; IMiD, immunomodulatory drug; IQR, interquartile range; LDH, lactate dehydrogenase; PI, proteasome inhibitor; R‐ISS, Revised International Staging System; R2-ISS, second revision of the International Staging System; t, translocation; ULN, upper limit of normal. |
|
|
Clinical characteristic, % (unless otherwise stated) |
All participants |
|---|---|
|
Median age (IQR), years |
62 (55─68) |
|
Age >65 years |
34.4 |
|
Sex |
|
|
Female |
38 |
|
Male |
62 |
|
LDH >ULN |
22.2 |
|
Cytogenetic abnormalities |
|
|
del(17p) |
15.4 |
|
1q1 |
52.5 |
|
t(4:14) |
13.9 |
|
R-ISS stage |
|
|
I |
13.9 |
|
II |
63.3 |
|
III |
22.8 |
|
First-line treatment |
|
|
IMiD |
27.8 |
|
PI |
64.6 |
|
IMiD+PI |
7.6 |
|
ASCT |
5.9 |
Median PFS and estimated OS for the real-world study cohort can be seen in Figure 2, according to R2-ISS stage, and relative to data from the EMN study. Compared with the EMN study, the real-world cohort was older (34.4% vs 23%), had a higher proportion of patients with ISS Stage III (49.6% vs 25%; p < 0.001), a higher proportion with elevated LDH (22.2% vs 16%; p = 0.002), and higher rates of cytogenetic anomalies, including del[17p] (15.4% vs 12%; p = 0.022) and 1q anomalies (52.5% vs 37%; p < 0.001), likely accounting for the lower median OS and PFS of this cohort.
Figure 2. Median OS and PFS for the EMN and real-world myeloma cohorts*
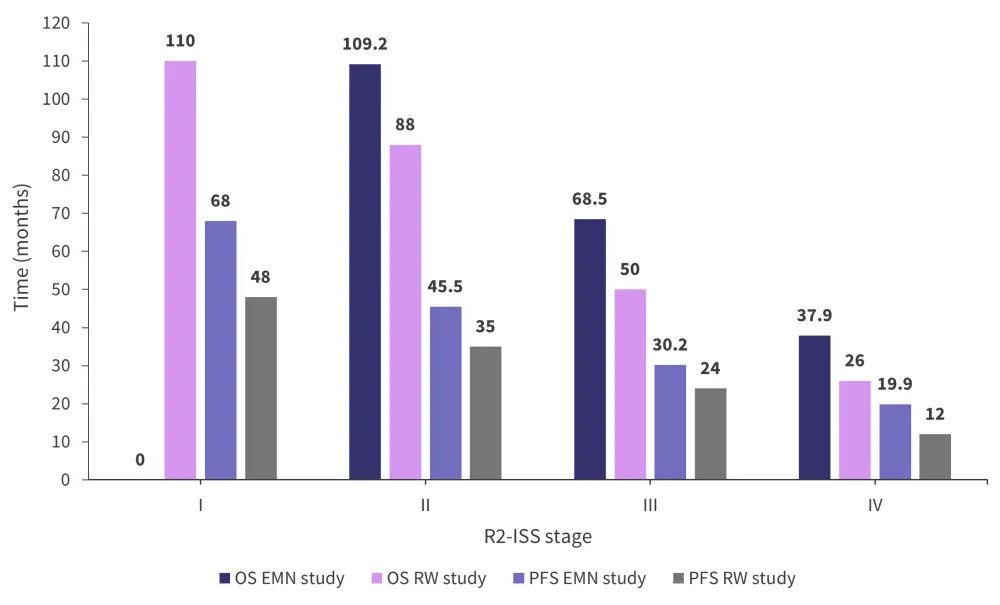
EMN, European Myeloma Network; OS, overall survival; PFS, progression-free survival; R2-ISS, second revision of the International Staging System; RW, real-world.
*Adapted from D’Agostino, et al.2 and Chen, et al.3
Patients were classified according to the R2-ISS described above, with the rates of each risk factor in each R2-SS visible in Table 4.
Table 4. Distribution of risk factors according to R2-ISS stage*
|
1q+, 1q gain/amplification; del, deletion; ISS, International Staging System; LDH, lactate dehydrogenase; R2‐ISS, second revision of the ISS; t, translocation. |
||||
|
Risk factor, % |
R2‐ISS I |
R2‐ISS II |
R2‐ISS III |
R2‐ISS IV |
|---|---|---|---|---|
|
No risk factors |
100 |
— |
— |
— |
|
ISS II |
— |
47 |
33 |
11 |
|
ISS III |
— |
— |
59 |
89 |
|
Elevated LDH |
— |
7 |
16 |
70 |
|
del(17p) |
— |
7 |
13 |
40 |
|
t(4;14) |
— |
7 |
11 |
31 |
|
1q+ |
— |
33 |
56 |
85 |
Adjusted for age, treatment, sex, and transplant status, R2‐ISS score was determined to be an independent prognostic factor, with an OS hazard ratio of 7.055 (95% confidence interval, 3.626–13.726; p < 0.001) for R2‐ISS IV vs I and 2.707 (95% confidence interval, 1.436–5.103; p = 0.002) for R2‐ISS III vs II. Higher Stage III and IV disease demonstrated significantly poorer outcomes in this population.
Conclusion
The R2-ISS is an updated and simple prognostic staging system, allowing a better stratification of patients with intermediate-risk NDMM into low-intermediate and intermediate-high risk. The potential of the four-tier classification includes avoiding the overtreatment of some intermediate-risk patients, and undertreatment of others, with both outcomes having potentially profound implications for patients with NDMM. The R2-ISS performed well in both the initial validation cohort and the replication study presented here, maintaining its prognostic power in a distinct patient population. Further large validation studies are needed to demonstrate this prognostic utility in other clinical settings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




