All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
The Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study
During the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, a number of presenters discussed the results of the Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study; a nationwide screening program for multiple myeloma (MM) and its precursors. The Multiple Myeloma Hub is happy to provide a summary of the key talks and posters presented on this study.
Study design
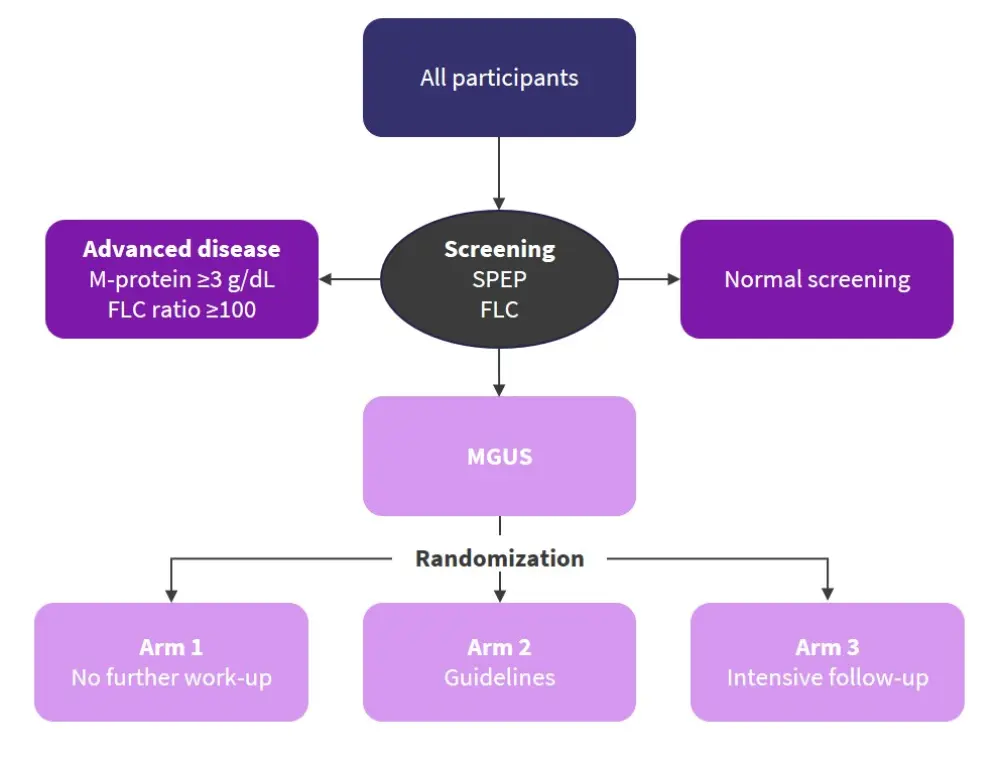
All residents of Iceland ≥40 years of age were invited to participate in the study. Of the 148,708 people who were eligible, 54.3% agreed to participate. So far, 75,422 people have been sampled. The study design is shown in Figure 1. Dynamic randomization was used to split patients between the three arms.1
Figure 1. Study design for screening of individuals with MGUS*
FLC, free light chain; MGUS, monoclonal gammopathy of undetermined significance; SPEP, serum protein electrophoresis.
*Adapted from Kristinsson1 and Thorsteinsdottir.2
In Arm 1, patients received traditional healthcare in the Icelandic system as normal. Arms 2 and 3 received full medical and clinical examinations along with blood work-up. In addition, Arm 3 also underwent whole-body low-dose computed tomography (WBLDCT) assessment, yearly follow-up, and more intense bone marrow follow-up.1
The primary aims of this study were1:
- To evaluate the impact of screening for monoclonal gammopathy of undetermined significance (MGUS)
- Map clinical and epidemiological characteristics of patients with smoldering MM (SMM)
- Obtain evidence to show which work-up and follow-up protocols are ideal
- Integrate biological, genetic, and imaging markers into a risk model for MM progression
- Assess impact on quality of life
- Biobanking
- Assess the value of early detection and treatment
An SMM diagnosis was reached when a patient exhibited 10−60% bone marrow plasma cells with or without ≥3 g/dL M-protein in the absence of MM defining events. The prevalence of SMM was estimated from Arm 3 of the trial as these patients underwent bone marrow testing and WBLDCT.2
Results
SMM screening2
In total, 3,725 of the 75,422 (5%) screened individuals produced abnormal results and were randomized to one of the three arms. SMM was diagnosed in 180 patients. Patient characteristics included:
- Males, 61%; females, 39%
- Median age, 70 years (range, 44−92 years)
- M-protein present, 84%, with a median of 0.5 g/dL (range, 0.01−3.5 g/dL)
- Abnormal free light chain (FLC) analysis only, 14%
- Abnormal FLC ratio, 64%
The IgG isotype was most common in this cohort. A normal FLC ratio and serum protein electrophoresis (SPEP) was seen in four patients despite abnormal screening results at the time of SMM diagnosis. However, since then, mass spectrometry results from three of these individuals has been received, which show lambda peaks and indicate patients were just below the limit of detection for FLC and SPEP. Most patients had a low plasma cell burden of 11−20% at diagnosis (Table 1).
Table 1. Characteristics of patients with SMM*
|
BMPC, bone marrow plasma cells; FLC, free light chain; Ig, immunoglobulin; MRI, magnetic resonance imaging; PET, positron emission tomography; SPEP, serum protein electrophoresis. |
|
|
Characteristic, % |
SMM (n = 180) |
|---|---|
|
Isotype (SPEP and FLC analysis) |
|
|
IgG |
57 |
|
IgA |
24 |
|
Biclonal |
3 |
|
Light-chain |
14 |
|
Normal FLC and SPEP analysis |
2 |
|
BMPCs |
|
|
11−20% |
73 |
|
21−30% |
18 |
|
31−40% |
4 |
|
41−50% |
5 |
|
Skeletal imaging |
|
|
MRI |
15 |
|
WBLDCT |
73 |
|
PET/CT |
1 |
|
Skeletal survey |
7 |
|
No imaging |
4 |
A total of 970 of the 1,279 patients who were randomized to Arm 3 had bone marrow samples taken. SMM was diagnosed in 10.8% of these patients. The prevalence of SMM was therefore estimated to be 0.53% (95% confidence interval [CI], 0.49−0.57%) in people aged ≥40 years.
In men the prevalence was higher at 0.7% (95% CI, 0.64−0.75%) compared with 0.37% in women (95% CI, 0.32−0.41%). The prevalence of SMM increased with age.
MGUS screening1
Figure 2 shows that increased levels of screening resulted in a significant rise in diagnoses of MM precursors, such as MGUS and SMM, after 3 years of follow-up (p < 0.001).
Figure 2. MGUS screening results*
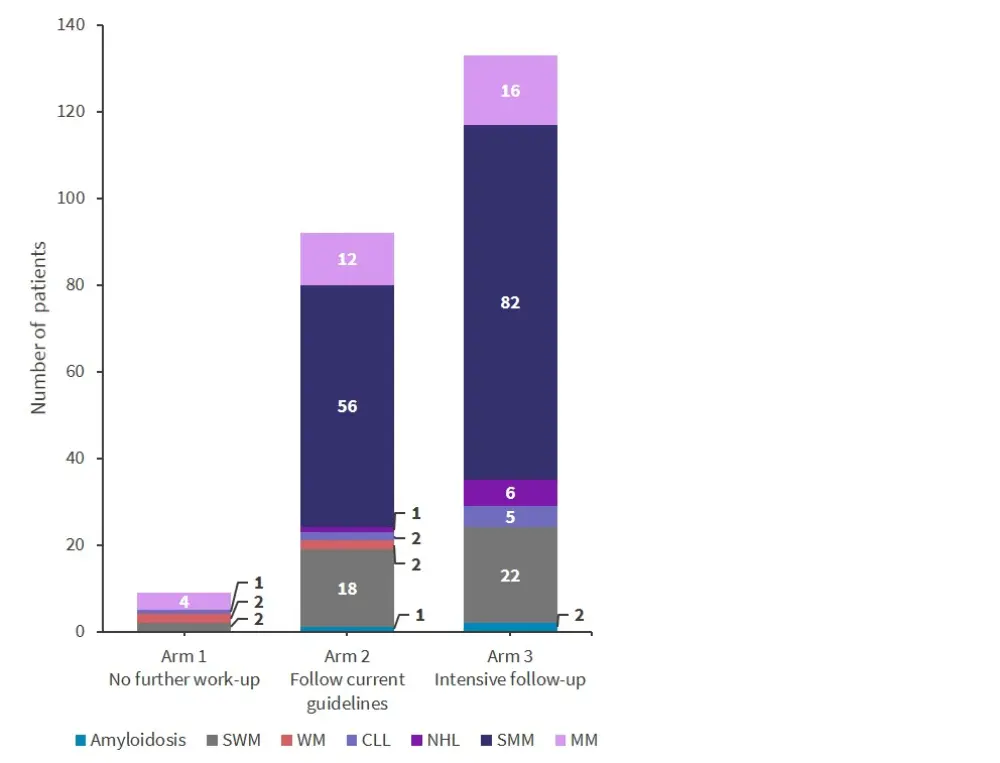
CLL, chronic lymphocytic leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; SMM, smoldering multiple myeloma; SMW, smoldering Waldenstrom’s macroglobulinemia; WM, Waldenstrom’s macroglobulinemia.
*Adapted from Kristinsson.1
When excluding indolent disease, the difference between arms remained statistically significant (p = 0.0046; Figure 3).
Figure 3. MGUS screening results excluding indolent disease*
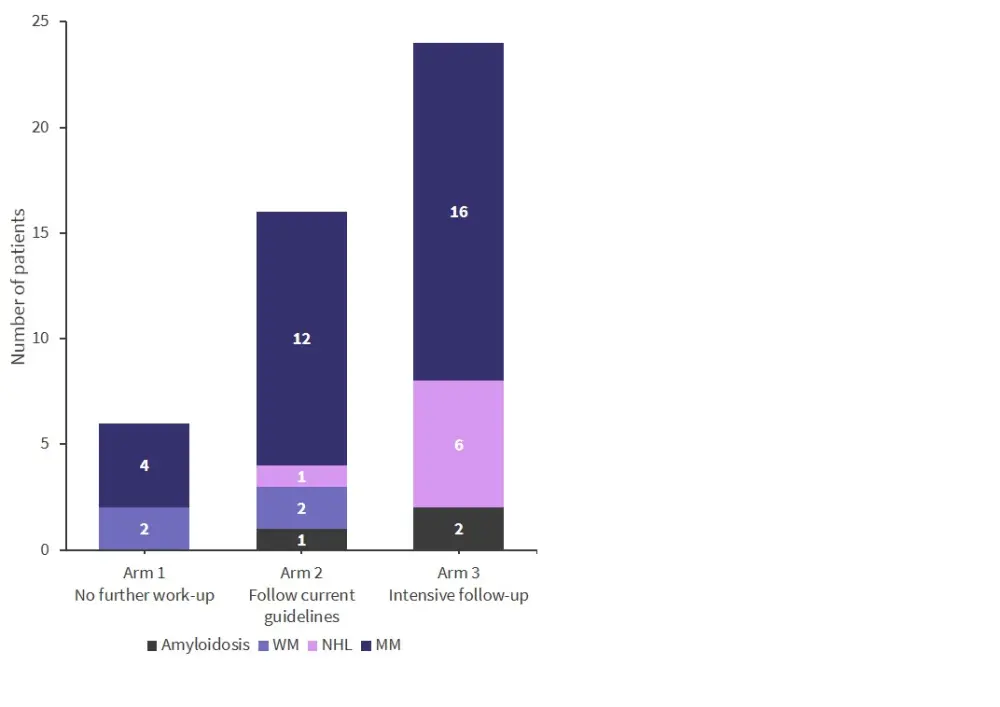
MM, multiple myeloma; NHL, non-Hodgkin lymphoma; WM, Waldenstrom’s macroglobulinemia.
*Adapted from Kristinsson.1
MGUS screening bias3
In a poster presented by Aðalbjörg Sigurbergsdóttir3 at ASH 2021 the selection bias in previous MGUS research was assessed and compared with screening in the iStopMM study. This substudy assessed the association of comorbidities, demographic-factors, and MGUS-related factors.
This study included data from 3,297 patients and the baseline characteristics of this subgroup are shown in Table 2. The patients with clinically diagnosed MGUS were found to be significantly older than those found during screening (p < 0.01). In addition, patients with clinically diagnosed MGUS were more likely to live in the capital area than those identified by screening (p < 0.05). Patients with MGUS identified by screening had a significantly lower M-protein concentration, at a mean of 0.33 g/dL, compared with clinically diagnosed patients (p < 0.001).
Table 2. Baseline characteristics for patients with clinical MGUS and MGUS by screening*
|
CI, confidence interval; MGUS, monoclonal gammopathy of unknown significance; SD, standard deviation. |
||||
|
Characteristic |
Clinical MGUS |
Screened MGUS |
p value |
Δ (95% CI) |
|---|---|---|---|---|
|
Median age, years (range) |
73 (42−97) |
69 (41−101) |
<0.01 |
— |
|
Male sex, % |
50.0 |
53.8 |
0.31 |
— |
|
Residence in the capital area, % |
64.7 |
56.4 |
<0.05 |
— |
|
Mean M-protein concentration, g/dL (SD) |
0.47 (0.38) |
0.33 (0.31) |
<0.001 |
0.14 (0.10−0.19) |
|
Mean number of comorbidities (SD) |
2.79 (2.10) |
2.09 (1.75) |
<0.001 |
0.60 (0.37−0.84) |
Clinically diagnosed patients with MGUS had a significantly increased number of comorbidities compared with patients with MGUS identified by screening (p < 0.001). Comorbidity classes that were significantly different (p < 0.001) in prevalence between the two cohorts included:
- Chronic kidney diseases
- Endocrine disorders
- Heart failure
- Neurological diseases
- Rheumatological diseases
Circulating plasma cell monitoring4
Further analysis was carried out using data from the iStopMM study to assess the value of monitoring circulating tumor plasma cells (CTPCs) in patients with MM precursor conditions. These data were presented as a poster by Jon Oskarsson4 at ASH 2021. CTPCs have received attention in recent years due to the minimally invasive nature of sampling. The aim for this study was to use CTPCs assessed by next-generation flow cytometry to monitor for disease progression in MM and precursor conditions.
So far, 189 patients have been included in this study (90 MGUS, 73 SMM, and 26 MM) and the frequency of CTPCs in these patients is illustrated in Figure 4. There was a significantly higher percentage of CTPCs in patients with SMM or MM compared with patients with MGUS (both p < 0.001).
Figure 4. CTPCs in peripheral blood*
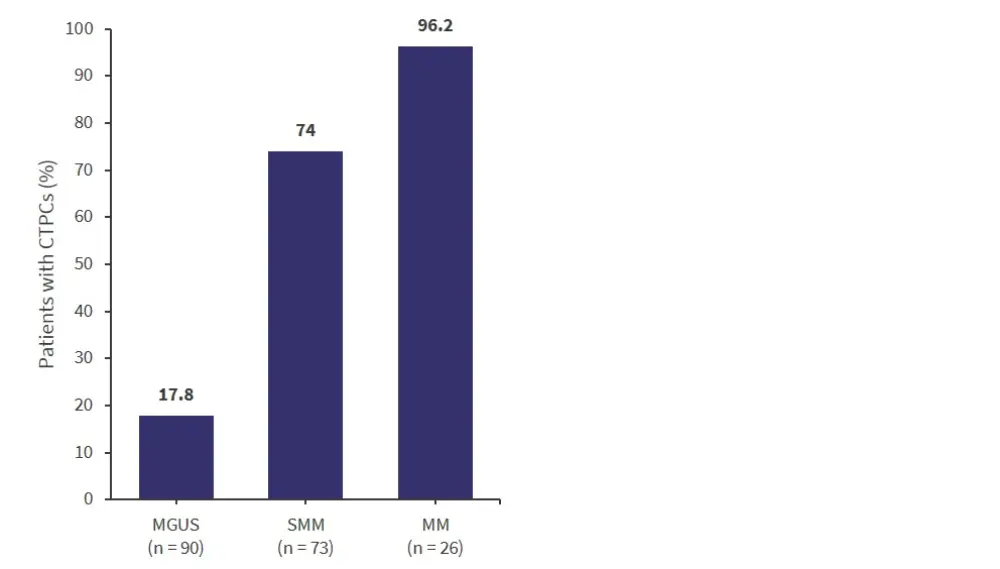
CTPCs, circulating plasma tumor cells; MGUS, monoclonal gammopathy of unknown significance; MM, multiple myeloma; SMM, smoldering multiple myeloma.
*Adapted from Oskarsson.4
CTPCs, circulating plasma tumor cells; MGUS, monoclonal gammopathy of unknown significance; MM, multiple myeloma; SMM, smoldering multiple myeloma.
*Adapted from Oskarsson.4
Median CTPC levels in peripheral blood in the different disease groups were:
- MGUS, 0 CTPC/µl
- SMM, 0.028 CTPC/µl (vs MGUS, p < 0.001)
- MM, 0.16 CTPC/µl (vs MGUS, p < 0.001; and vs SMM, p < 0.01)
In the MGUS and SMM groups, patients who were CTPC-positive had an increased percentage of tumor plasma cells (TPCs) in their bone marrow compartment compared with CTPC-negative patients. Of the patients in the SMM group with >95% TPCs in their bone marrow, 96% also had a measurable CTPC population in the peripheral blood. Patients with MGUS who were CTPC-positive had a significantly elevated level of serum monoclonal component compared with patients who were CTPC-negative (6.3 g/L vs 3.1 g/L, respectively; p < 0.01). The percentage of TPCs out of bone marrow plasma cells was also significantly increased in patients with CTPC-positive MGUS and SMM compared with CTPC-negative MGUS and SMM (both p < 0.001).
Conclusion
The iStopMM study is the largest screening program for MM and its publication at ASH 2021 was eagerly anticipated. Some key points from this study were that the prevalence of SMM found in this study was found to be surprisingly high at 0.53% in adults ≥40 years. Increased screening led to increased diagnosis of MGUS and other hematologic conditions. Even removing indolent disease, the difference between the study arms remained significant. Selection bias was identified in previous MGUS research, with patients more likely to be identified with MGUS if they live in Iceland’s capital city, have other comorbidities, and were older. Increased CTPC were found to be associated with SMM and MM compared with MGUS.
Further data are awaited on this trial to cover the psychological impact of screening and the development of a risk of progression model. At this point, Kristinsson did not recommend screening for MGUS until all the final results have been collected for this study.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

