All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
The DREAMMs at ASCO — updates on belantamab mafodotin added to MM backbone therapies
Despite significant improvements in the outcomes of patients with multiple myeloma (MM) in recent years, there is still a need for novel agents that specifically target myeloma cells, are well tolerated, and induce deep and durable responses. One of such novel agents is belantamab mafodotin (belamaf, GSK2857916), a first-in-class, antibody-drug conjugate against B-cell maturation antigen linked to cytotoxic monomethyl auristatin F. Belamaf is being evaluated in the DREAMM program consisting of several clinical trials, where belamaf is used as a single agent or in combination with other treatments in newly diagnosed or heavily pretreated patients with MM.
Recently, we summarized the data from the trials using belamaf as a single agent. Here is a summary of new data from the DREAMM studies using belamaf in combination with other agents presented during the 2020 virtual meeting of the American Society of Clinical Oncology (ASCO).
DREAMM-61,2
Study design1
The potential to further improve outcomes for patients with relapsed/refractory MM (RRMM) treated with single-agent belamaf by combining it with standard of care regimens is being investigated in the ongoing DREAMM-6 study. A poster describing the study design was presented at the ASCO20 meeting. DREAMM-6 (NCT03544281) is an open-label, phase I/II study in patients with RRMM previously treated with ≥ 1 prior line of therapy. The study is divided into two parts:
- Dose escalation phase to examine the safety, tolerability, and pharmacokinetics (PK) of
- belamaf monotherapy (1.9, 2.5, and 3.4 mg/kg)
- combination of belamaf administered as a single (Day 1) or a split dose (divided equally on Days 1 and 8) with lenalidomide/dexamethasone (B-Rd) administered on a 28-day cycle
- combination of belamaf administered as a single (Day 1) or a split dose (divided equally on Days 1 and 8) with bortezomib/dexamethasone (B-Vd) administered as a 21-day cycle
- Expansion phase to further evaluate the safety, PK, and preliminary efficacy of belamaf combinations at the dose levels and schedules defined in part 1 and establish the recommended dose and schedule for further studies.
B-Rd is administered until disease progression, intolerance, consent withdrawal, or death; B-Vd is administered in up to 8 cycles, followed by belamaf monotherapy until disease progression, intolerance, consent withdrawal, or death.
The key eligibility criteria included
- Age ≥ 18 years
- Histologically or cytologically confirmed MM
- Measurable disease
- ≥ 1 prior line of therapy
- Adequate organ system function
- Eastern Cooperative Oncology Group (ECOG) performance status 0–2
- No monoclonal antibody therapy in the last 30 days
Preliminary results from the B-Vd arm as of data cut-off date of March 30, 20202
The safety and tolerability of the B-Vd combination of the DREAMM-6 study were selected as an oral abstract at ASCO20 and presented by Ajay Nooka from Emory University, US. The reported data included dose escalation and cohort expansion parts, for 2.5 mg/kg belamaf administered as a single (Day 1) or a split dose (divided equally on Days 1 and 8), in combination with Vd.
In the dose escalation part of the study, no dose-limiting toxicities were observed with a single 2.5 or 3.4 mg/kg doses of belamaf. To date, 59 patients have been treated with B-Vd including, 18 patients who received 2.5 mg/kg single dose of belamaf every 3 weeks (Table 1).
Table 1. Patients and baseline disease characteristics
|
B-Vd, belantamab mafodotin (single dose 2.5 mg/kg)/bortezomib/dexamethasone; ECOG, Eastern Cooperative Oncology Group performance status; ISS, International Staging System *Defined as t(4;14), t(4;16), or del17p13; cytogenetic data not available for six patients |
|
|
Characteristic |
B-Vd (n = 18) |
|
Median age (range), years |
67 (47–83) |
|
Male gender, % |
61 |
|
Number of prior therapies, % 1 2–3 4–6 ≥ 7 Median (range) |
22 33 22 22 3 (1–11) |
|
ISS stage, % I II III Unknown |
22 44 17 17 |
|
ECOG, % 0–1 ≥ 2 |
83 17 |
|
High-risk cytogenetics, %* |
33 |
- Median weeks of treatment at the data cut-off was 18.2 (range, 6.0–46.4)
- The overall response rate was 78% (95% CI, 52.4–93.6), including 50% of patients who achieved very good partial response (Figure 1)
- Duration of response was not yet reached
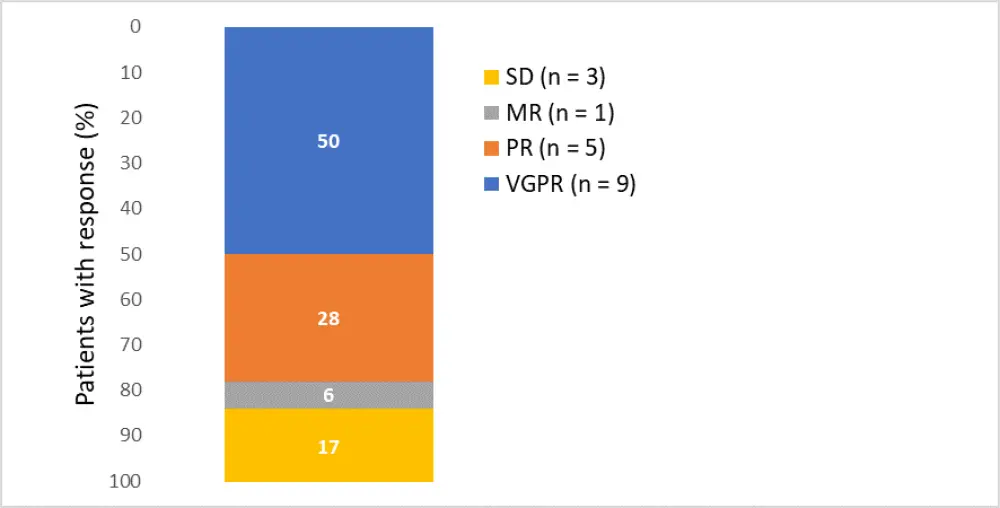
Figure 1. Best confirmed response in patients receiving belamaf 2.5 mg/kg single dose plus Vd. MR, minimal response; PR, partial response; SD, stable disease; VGPR, very good partial response
- The reported adverse events (AEs) included
- infusion-related reactions (n = 3 of Grade 2)
- keratopathy (n = 1, 7, and 10 of Grade 1, 2, and 3, respectively)
- thrombocytopenia (n = 1, 3, and 8 of Grade 2, 3, and 4 respectively)
- To date, five patients permanently discontinued treatment due to AEs, but none related to belamaf (Table 2)
Table 2. Overview of AEs
|
AEs, adverse events; belamaf, belantamab mafodotin; B-Vd, belantamab mafodotin (single dose 2.5 mg/kg)/bortezomib/dexamethasone |
|
|
Patients with AE, % |
B-Vd (n = 18; part 1 + part 2) |
|
AEs related to study treatment Grade 3/4 AEs leading to permanent discontinuation of a study treatment AEs leading to permanent discontinuation of belamaf |
100 89 28 0 |
|
AEs leading to dose reductions Keratopathy Thrombocytopenia |
72 39 33 |
|
AEs leading to dose interruption/delay Keratopathy Thrombocytopenia |
100 83 39 |
|
Any serious AEs Serious AEs related to study treatment |
67 28 |
In conclusion, the preliminary data of the B-Vd suggest that the combination administered as a single 2.5 mg/kg dose has a manageable safety profile consistent with that reported for single agents. The activity of the combination is higher than previously demonstrated for Vd or belamaf 2.5 mg/kg dose (overall response rate of 78% vs 50–63% and 32%, respectively) in this setting. The study is ongoing, and a longer follow-up will establish the duration of response and whether the response will deepen over time.
Other trials from the DREAMM program reported at ASCO20
DREAMM-53
The design of the DREAMM-5 (NCT04126200) was presented by Paul Richardson, Dana-Farber Cancer Institute, UK. This phase I/II platform trial utilizes a master protocol that will enable sub-studies to evaluate a potential synergy of belamaf combined with other agents, with other mechanisms of action in patients with RRMM. The sub-studies currently open or opening soon to enrollment include belamaf combinations with
- GSK3174998, an OX40 agonist antibody
- GSK3359609, an ICOS agonist antibody
- Nirogacestat, a selective γ-secretase inhibitor
Key inclusion criteria include
- Age ≥ 18 years
- Histologically or cytologically confirmed MM
- Measurable disease
- ECOG 0–2
- ≥ 3 prior lines of therapy (including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody)
- Acceptable hematologic function
Study endpoints are presented in Table 3.
Table 3. Endpoints for dose exploration and cohort expansion phases of DREAMM-5
|
ADAs, antidrug antibodies; AEs, adverse events; AESI, AE of special interest; CBR, clinical benefit rate; DLT, dose-limiting toxicity; DoR, duration of response; HRQoL, health-related quality of life; IV, intravenous; MRD, minimal residual disease; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PK, pharmacokinetics; TTP, time to progression; TTR, time to recurrence; VGPR, very good partial response |
|||
|
Phase |
Primary endpoints |
Secondary endpoints |
Exploratory endpoints |
|
Dose exploration phase ≤ 10 patients per dose level |
- DLT - AEs |
- ORR - Drug concentrations - ADAs against IV treatments - AESIs, ocular findings |
- PK - CBR, DoR, TTR, PFS, OS - Biomarkers - Pharmacogenomics - MRD negativity in patients with ≥ VGPR |
|
Cohort expansion phase ≥ 35 patients per each combination |
- ORR |
- CBR, DoR, TTR, PFS, OS - AEs, AESIs, ocular findings - ADAs against IV treatments - drug concentrations |
- PK - HRQoL - Biomarkers - Pharmacogenomics - MRD negativity in patients with ≥ VGPR |
DREAMM-9
Saad Usmani, Levine Cancer Institute, UK, presented the design of the DREAMM-9 study evaluating the efficacy and safety of bortezomib/lenalidomide/dexamethasone (VRd) vs belamaf plus VRd in transplant-ineligible newly diagnosed MM (NCT04091126). This phase III, open-label, randomized multicenter study, is currently recruiting patients. Like other studies in the DREAMM program, it will consist of a dose escalation part and the cohort expansion part.
The first part aims to recruit ≤ 12 patients per dose cohort, with an additional six patients in the cohort most likely to be selected as a recommended dose for the second part of the study. Similar to the DREAMM-6 study, single and split doses of belamaf 2.4 mg/kg and 3.4 mg/kg will be investigated. In the second part of the study, approximately 750 patients will be randomized 1:1 to VRd ± belamaf. VRd combination with belamaf will be given for 8 cycles, which will be followed by belamaf plus Rd until disease progression or unacceptable toxicity.
Key inclusion criteria include
- Age ≥ 18 years
- Ineligible for high dose chemotherapy and allogeneic hematopoietic stem cells transplantation
- Confirmed MM with measurable disease
- ECOG 0–2
- ≥ 3 prior lines of therapy (including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody)
- Adequate organ function
The endpoints include:
- Primary
- Dose escalation part: dose-limiting toxicities and AEs
- Cohort expansion part: MRD-negativity rate and PFS
- Secondary
- Dose escalation part: relative dose intensity of lenalidomide and bortezomib after 4 cycles of belamaf + VRd, cumulative administered dose of belamaf after 4 cycles of belamaf + VRd, PK, and anti-drug antibodies incidence
- Cohort expansion part: efficacy, safety, belamaf exposure, anti-drug antibodies incidence, and health-related quality of life
Conclusion
The DREAMM program is comprehensive and consists of several studies evaluating belamaf as a monotherapy and in combination with other agents. The studies are mostly carried out in the RR setting, with the exception of the DREAMM-9, which will enroll newly diagnosed transplant-ineligible patients with MM. Other studies are designed to further improve the efficacy and safety of belamaf alone and in combinations. The most frequent reported AEs include keratopathy and thrombocytopenia, which can be successfully managed with dose adjustments and resolve after treatment.
The DREAMM-3 trial design (NCT04162210, belamaf + pomalidomide) and preliminary results from DREAMM-4 (belamaf + pembrolizumab) in RRMM, will be presented during the 25th annual meeting of the European Hematology Association (EHA), so stay tuned in.
Expert Opinion
 Rakesh Popat
Rakesh PopatReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

