All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Smoldering MM: Management in 2022 and beyond
Do you know... According to the SMM management guidelines outlined at ASH 2022, what are the criteria for high-risk SMM?
Smoldering multiple myeloma (SMM) is a heterogeneous, asymptomatic, and rarely diagnosed precursor condition proceeding multiple myeloma (MM).1,2 It represents an intermediary state between monoclonal gammopathy of undetermined significance and MM.1
The International Myeloma Working Group (IMWG) SMM diagnostic criteria are as follows1:
- Serum IgA/IgG ≥3 gm/dL, or urine IgA/IgG ≥500 mg per 24 hours and/or 10–60% clonal bone marrow plasma cells
- Absence of MM defining events/amyloidosis
No single molecular or pathologic feature can distinguish reliably between patients with monoclonal gammopathy of undetermined significance or MM; therefore, specific risk factors are used to identify patients with SMM in whom malignant transformation is likely to have occurred (high-risk SMM) for the purposes of clinical practice and clinical trials.1
Here, we summarize current SMM risk stratification and treatment recommendations and review potential developments on the horizon based on ongoing clinical trials in SMM as presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition.
SMM risk stratification and management
Debate is ongoing regarding the need for treatment in patients with SMM.2 Some experts advocate close surveillance without treatment initiation regardless of risk status, stating the potential for overtreatment and secondary malignancies with lenalidomide as reasons for observation only. However, others advocate early treatment initiation for high-risk patients and selected low-risk patients.2
Some of the discrepancy between expert opinions on the need for treatment stems from the lack of risk stratification consensus, with criteria varying between models (Table 1) and overall agreement between models at only around 50%.2
Table 1. SMM risk stratification models*
|
BMPC, bone marrow plasma cell; FISH, fluorescent in situ hybridization; FLC, free light chain; IWMG, International Myeloma Working Group; MFC, multiparameter flow cytometry; PC, plasma cell; SMM, smoldering multiple myeloma. |
|
|
Risk stratification model |
Stratification criteria |
|---|---|
|
Mayo Clinic 2007 |
Risk stratification into 3 groups based on serum M protein and BMPCs |
|
PETHEMA 2007 |
Risk stratification according to aberrant PCs by flow cytometry and immunoparesis |
|
Mayo Clinic 2018 |
Risk stratification into 3 groups based on serum M protein and BMPCs |
|
Risk stratification based on combined risk score derived from FLC ratio, M protein, BMPCs, and FISH |
|
Despite ongoing debate regarding risk stratification, it is generally agreed that high-risk SMM has ∼50% risk of progression to MM at 2 years from SMM diagnosis.2
In a recent publication, Rajkumar et al.1 reported that in randomized trials conducted in specialized centers, a 90% reduction in end organ damage can be achieved for patients with high-risk SMM when treated with lenalidomide/lenalidomide + dexamethasone therapy versus observation alone and delayed therapy, suggesting significant benefit of early intervention in these patients.1,2
Rajkumar et al.1 recommend that patients newly diagnosed with high-risk SMM (defined as any two of bone marrow plasma cells >20%, serum monoclonal protein >2 gm/dL, serum free light chain ratio >20, or a high-risk score based on the IMWG SMM scoring system) should be offered lenalidomide or lenalidomide plus dexamethasone therapy for 2 years or clinical trial enrolment. Those with low-risk SMM should be observed on a 3–4 monthly basis without therapy, except in cases of evolving M protein and hemoglobin changes, where early intervention should be considered (Figure 1).1
Figure 1. SMM management approach*
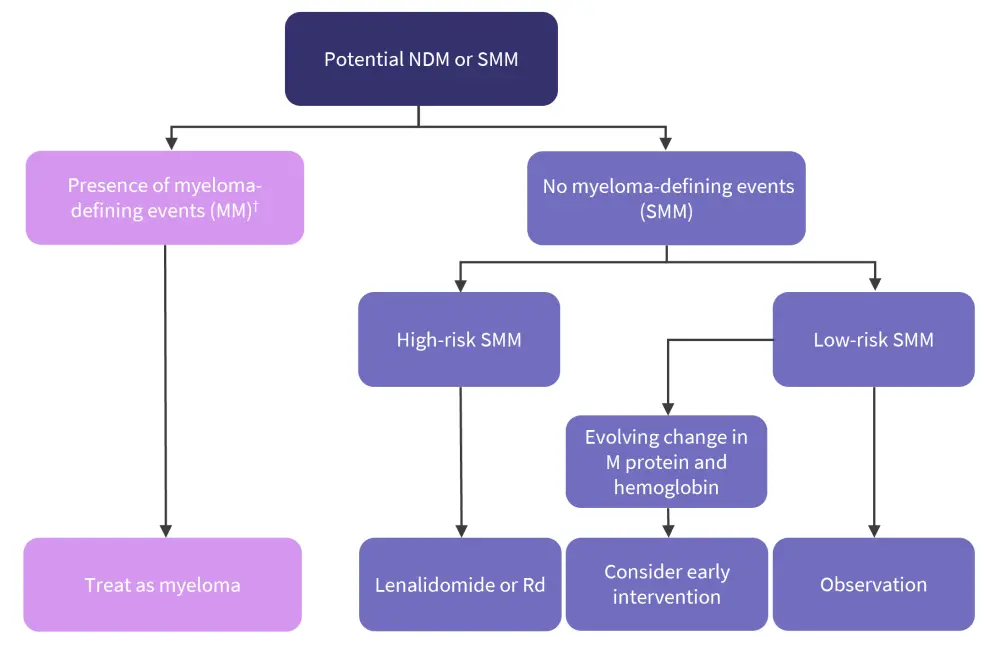
MM, multiple myeloma; NDM, newly diagnosed myeloma; Rd, lenalidomide + dexamethasone; SMM, smoldering multiple myeloma.
*Adapted from Rajkumar, et al.1
†MM related end organ damage (hypercalcemia, anemia, light chain cast nephropathy, osteolytic bone lesions), serum free light chain ratio ≥100 with involved serum free light chain level ≥100 mg/dL and urine monoclonal protein ≥200 mg/24 hours, ≥60% clonal bone marrow plasma cells, >1 focal lesion.
Phase III trials of lenalidomide treatment in patients with high-risk SMM have demonstrated improved progression-free survival (PFS) and overall survival (OS) versus observation only (QUIREDEX trial; NCT00480363) and improved PFS (but not improved OS; Eastern Cooperative Oncology Group E3A06 trial; NCT01169337).2,3 However, both trials confirmed treatment-related toxicities (with one treatment-related death in each trial) and increased rates of secondary cancers.2,3
In the ASH 2022 educational session entitled “The Consultant’s Guide to Smoldering Multiple Myeloma”, Thorsteinsdottir2 summarized the following recommendations for SMM management based on current knowledge:
- Work-up at diagnosis:
- Complete blood count, creatinine, calcium, blood and urine protein electrophoresis with immunofixation, and free light chain analysis
- Bone marrow evaluation with fluorescent in situ hybridization and flow cytometry where possible
- If whole-body low-dose computed tomography is negative, use whole-body magnetic resonance imaging, spinal magnetic resonance imaging, or fluorodeoxyglucose-positron emission tomography/computerized tomography
- Risk stratification according to risk of progression:
- Mayo Clinic 2018 model (2-20-20)/IMWG 2020 model (Table 1) or evaluation of evolving disease
- Exclusion of other plasma cell-associated conditions
- Inform the patient of follow-up and treatment initiation strategies and discuss the patient’s wishes
- No need for immediate management decisions
The current management guidelines summarized in the session recommend close monitoring for low and intermediate-risk SMM and very close monitoring with potential treatment initiation for high-risk patients (Figure 2).
Figure 2. SMM management guidelines presented at ASH 2022*
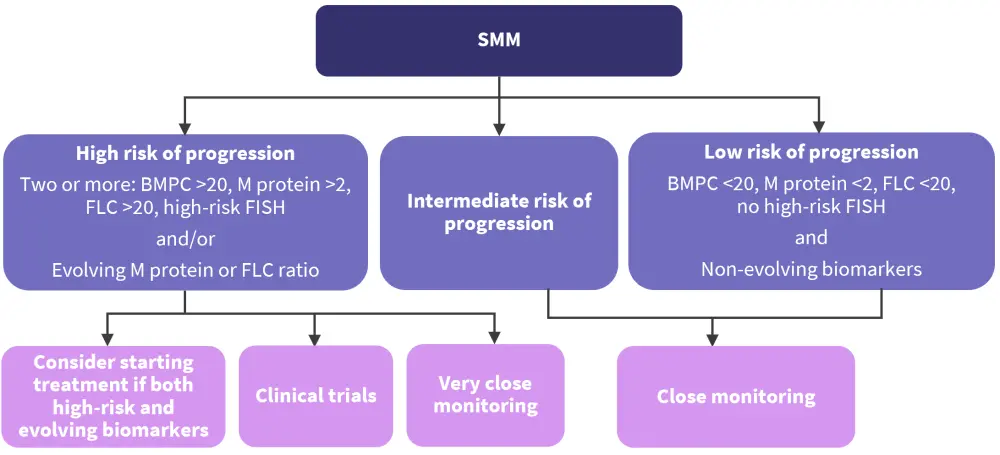
ASH, American Society of Hematology; BMPC, bone marrow plasma cells; FISH, fluorescent in situ hybridization; FLC, free light chain; SMM, smoldering multiple myeloma.
*Adapted from Thorsteinsdottir.2
Ongoing trials presented at ASH 2022
Curative strategy for high-risk SMM: GEM-CESAR MRD analysis3
Mateos3 presented a post hoc analysis of sustained undetectable measurable residual disease (MRD) from the phase II GEM-CESAR trial (NCT02415413) designed to evaluate the efficacy and toxicity of an intensive therapeutic approach in patients with asymptomatic high-risk SMM (N = 90; Figure 3).
Figure 3. GEM-CESAR study design*
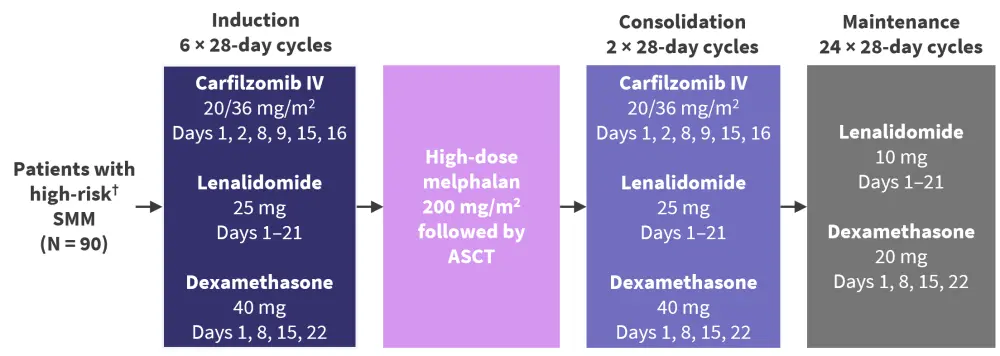
ASCT, autologous stem cell transplant; MM, multiple myeloma; SMM, smoldering multiple myeloma.
*Adapted from Mateos.3
†High risk defined according to Mayo Clinic/PETHEMA model.
- Primary endpoint: MRD-negative rate (by flow cytometry) after induction, autologous stem cell transplant consolidation/maintenance, and 3 and 5 years after maintenance
-
Secondary endpoints: Response, time to progression, PFS, OS, safety
The MRD-negative rate results are shown in Table 2.
Table 2. GEM-CESAR MRD-negative results in evaluable patients*
|
HDT-ASCT, high-dose therapy and autologous stem cell transplantation; MRD, measurable residual disease. |
||
|
Characteristic, % |
3 months post HDT-ASCT |
4 years post HDT-ASCT |
|---|---|---|
|
MRD negative at 10−5 |
68.3 |
43 |
|
MRD negative at 10−6 |
48 |
48 |
At 70 months post treatment, 34 patients (38%) had a biochemical progression, while only 5 (6%) had progressed to overt MM. Achievement of MRD negativity was a clear predictive factor; 80% of patients who achieved MRD negativity at the end of 2 years of maintenance remained biochemical-progression-free 3 years after, versus 42% of patients who were MRD positive. However, it is important to note that 88% of these MRD-positive patients had not progressed to active MM either. These results suggest that a curative approach for high-risk SMM needs further follow-up and analysis, especially to accurately identify those patients at a real higher-risk of progressing to MM.
Fixed duration therapy for high-risk SMM: ASCENT4
Kumar4 presented results from the phase II ASCENT trial (NCT03289299) designed to test the hypothesis that intense therapy initiated during the SMM precursor phase may be able to eradicate the malignant clone and thereby potentially lead to long-term remissions or even cure. The trial tested the efficacy of a 2-year fixed duration transplant-free regimen (N = 87; Figure 4).
Figure 4. ASCENT study design*
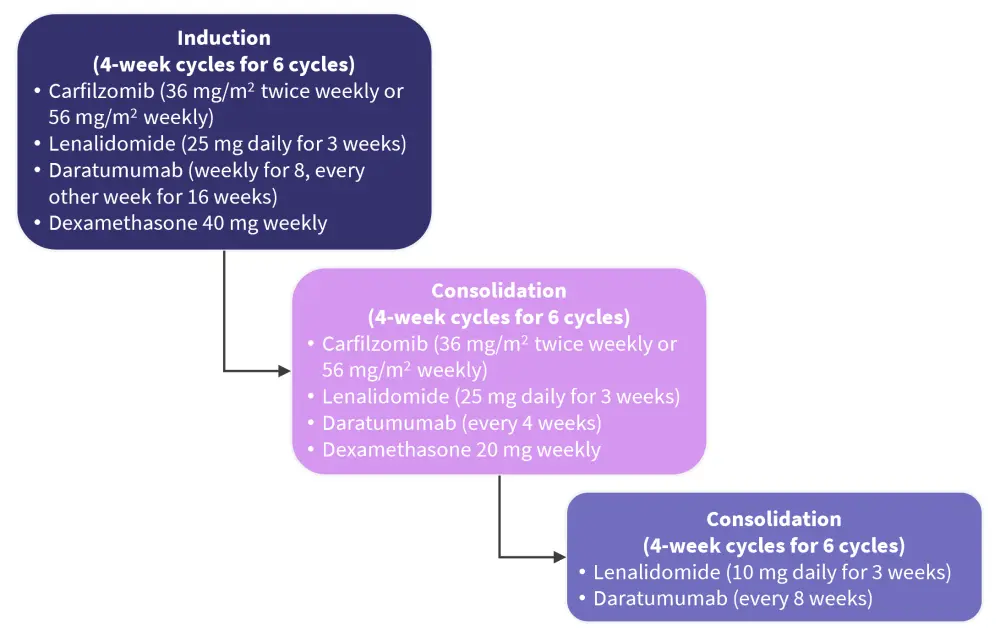
Adapted from Kumar.4
- Primary endpoint: Rate of confirmed stringent complete response
- Secondary endpoints: Safety, PFS, OS, MRD negativity rate at the end of consolidation
The combination of daratumumab, carfilzomib, lenalidomide, and dexamethasone given for a fixed duration of 2 years yielded high response rates (best overall response rate, 97%; very good partial response rate, ≥92%) and deep responses, including high rates of MRD negativity (bone marrow MRD negative, 84%; IMWG MRD negative, 64%). Responses appear durable, as indicated by sustained MRD negativity and the 90% PFS rate at 3 years. Almost 50% of patients are still receiving therapy, with depth of responses expected to improve. Any grade toxicity was observed in 92% of patients, with Grade ≥3 hematologic toxicity in 18% of patients and Grade ≥3 non-hematologic toxicity in 51% of patients).
Conclusion
Expert opinions on SMM treatment strategies remain divided, with a lack of consensus regarding treatment initiation in high-risk patients. This uncertainty stems from inter-model risk stratification variability and differing opinions on treatment risk/benefit ratios; for example, the risk of overtreatment, toxicities, and secondary malignancies versus undertreatment, resulting in organ damage, reduced PFS/OS, and poorer outcomes.
The guidelines presented at ASH 2022 recommend close monitoring for low and intermediate-risk SMM and very close monitoring for high-risk SMM, with the potential for clinical trial enrolment or treatment initiation.
Two phase III trials have demonstrated reduced risk progression to active MM when high-risk SMM is treated with lenalidomide/lenalidomide + dexamethasone versus observation alone, with one of the studies also demonstrating improved OS. Two phase II trials have demonstrated high response rates and durable MRD responses when intense combination therapy (with or without transplant) is initiated in SMM. Whilst outcomes may be improved with therapy in high-risk SMM, treatment-related toxicities and risk of secondary cancers remain important considerations in developing future treatment guidelines.
Many questions regarding treatment in high-risk SMM remain unanswered, and there is a need for trials with OS and quality of life as primary endpoints. Validated risk assessment models that consider evolving disease will be important in reaching a treatment consensus.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?




