All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Should anti-CD38 antibodies be used in all patients as induction therapy prior to transplant?
During The 6th World Congress on Controversies in Multiple Myeloma (COMy), Francesca Gay, University of Torino, Torino, IT, and our Steering Committee member Mohamad Mohty, Saint-Antoine Hospital and Sorbonne University, Paris, FR, debated on whether anti-CD38 antibody-based induction therapy should be used in all patients with multiple myeloma (MM) prior to transplant. They reviewed the data from various studies to address the issue, highlighting the CASSIOPEIA and GRIFFIN trials, and focused on daratumumab, which was the only approved anti-CD38 antibody up until recently.1
The Multiple Myeloma Hub has been focusing on the ongoing potential of monoclonal antibodies in MM as an editorial theme; find more information on these agents discussed here.
For
Mohamad Mohty started his discussion by describing the historic data which showed the importance of induction therapy prior to transplantation for patients with MM:
- Three major randomized trials showed the superiority of bortezomib, thalidomide, and dexamethasone (VTD) over the induction with the doublets TD or VD, but the complete response (CR) rates were only between 20–30% depending on the trial and the number of cycles
- VTD induction proved to be highly effective and highly popular and was approved in most countries. It was particularly beneficial in terms of progression-free survival (PFS) and PFS2 as shown in the GIMEMA study (PFS2 at 5 years of 76% VTD vs 63% TD, p = 0.0124)
- Other popular treatment regiments were bortezomib, cyclophosphamide, and dexamethasone (VCD) based on the phase II data of a small retrospective series, in which the CRs were quite modest (30–39% including near CR data)
- The IFM 2013-04 study compared VTD with VCD and showed a good response compared with the historic trial, with CRs of 13% and 10%, respectively. However, these response rates were not ideal
- The IFM 2009 trial used bortezomib, lenalidomide, and dexamethasone (VRD) as a backbone for induction or consolidation, and showed VRD as a highly effective induction regimen for patients with newly diagnosed MM (NDMM), achieving CR rates that were closer to 30%
- The SWOG S0777 trial, a randomized phase III trial, compared VRD with RD and showed VRD to be a powerful induction regimen
For the last 3 years, triple VD-based regiments have been the standard of care for induction therapy, but recent additions of anti-CD38 antibodies to these triple regimens have further increased CR rates.
- The stringent CR rates of the CASSIOPEIA study showed a significant improvement in favor of adding daratumumab to VTD induction (HR, 1.6; p = 0.001) with no additional safety concerns
-
- This translated into a better PFS (HR, 0.47; p < 0.0001)
- Dara-VTD has been approved by the U.S. Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) for induction therapy in young patients with NDMM
- In the randomized phase III, multicenter, GRIFFIN trial, stringent CR rates showed a significant improvement in favor of adding daratumumab to VRD induction, 42% vs 32%, Dara-VRD vs VRD, respectively
The addition of daratumumab to induction therapies resulted in deeper, better quality responses. Mohamad Mohty argues that these are the main reasons to defend that anti-CD38 antibody-based induction therapy should be used in all patients with MM prior to transplant, especially as there are no additional safety concerns.
Against
Francesca Gay began by explaining that if we want to see if it is worth using anti-CD38 antibodies as induction therapy, then we must first consider the goals of induction therapy; which are to achieve a rapid and deep response with a tolerable regimen that allows for adequate stem-cell collection.
Efficacy
Randomized trials using four cycles of the current standard of care treatment; proteasome inhibitors in combination with immunomodulatory agents such as VCD, VTD, and VRD, all showed deep responses (≥ very good partial response rates) of about 30–60%.
When a fourth agent, the anti-CD38 antibody daratumumab, was added, the rate of deep responses increased to about 65-73% in the case of VTD and VRD based quadruplets.
Furthermore, triplet combinations of the second-generation proteasome inhibitor, carfilzomib, in combination with lenalidomide and dexamethasone (KRD) could also provide a 70% ≥ very good partial response rates, suggesting anti-CD38 therapy may no longer be required.
However, Francesca Gay noted that these comparisons were limited because they compare data from different trials. Therefore, she focused on the CASSIOPEIA and GRIFFIN studies, which directly compared triplet combinations with and without daratumumab. These studies showed a higher rate of high-quality responses, specifically at post-consolidation, but also after induction with the quadruplet combination compared with the triplet.
Tolerability
The rate of Grade 3/4 hematologic toxicities was higher in the quadruplet combinations compared with triplet combinations:
- Thrombocytopenia – CASSIOPEIA: 7% vs 11% VTD vs Dara-VTD; GRIFFIN: 8% vs 16%, VRD vs Dara-VRD
- Neutropenia – CASSIOPEIA: 15% vs 28% VTD vs Dara-VTD; GRIFFIN: 15% vs 31%, VRD vs Dara-VRD
However, the Grade 3/4 infections and lymphopenia were comparable across the arms. Therefore, suggesting that the regiments were manageable.
Stem-cell collection
These trials also showed that a similar number of stem cells could be collected from both the triplet or quadruplet combinations, and the neutrophil engraftment was similar. However, the quadruplet combinations required a higher use of Plerixafor (CASSIOPEIA: 7% vs 22% VTD vs Dara-VTD; GRIFFIN: 55% vs 70%, VRD vs Dara-VRD)
Francesca Gay then made further points that it may be unnecessary to use anti-CD38 antibodies for all patients:
- As some patients can achieve a high-quality response without using the anti-CD38 antibody as demonstrated in the control arms of CASSIOPEIA and GRIFFIN trials
- The cost of a 4-drug regimen might be a drawback, as well as the additional use of Plerixafor
- The addition of daratumumab in patients with high-risk features is not enough to overcome the adverse prognosis of this subgroup
- Although treatment with anti-CD38 antibodies and other drug combinations, such as those used in the MASTER trial, may be beneficial for high-risk patients. To assess this, further randomized trials are required with larger numbers of patients
She then debated whether a combination of all the most effective drugs should be used at first-line treatment or whether another treatment strategy would be better to optimize patient outcomes.
- In the CASSIOPEIA and ALCYONE studies, the PFS of patients achieving minimal residual disease (MRD) negativity was similar regardless of if daratumumab was used or not
- Also, patients that achieved MRD negativity also relapsed, therefore, a cure is still not achievable by using a combination of all the most effective drugs as first-line treatment
- When anti-CD38 antibody is given at first relapse, there is an improvement in survival outcomes, as well as a high rate of MRD negativity that is sustained/prolonged. Therefore, if anti-CD38 antibodies are not used as first-line treatment they can be effectively used at first-relapse
- This may be important as 60% of all patients relapse and require second-line treatment, and the rate of relapse is likely to be higher in the younger population (as they have a longer life expectancy compared with elderly patients)
- Therefore, a better strategy should probably involve one that is more MRD or risk driven
Conclusion
The debaters agreed that the use of anti-CD38 antibodies as induction regiments seem to be beneficial. However, more data is required to determine if this is beneficial for all patients or for selected subgroups; and whether their use would have a better outcome at the relapse setting for different subsets of patients with MM.
In a poll conducted by the Multiple Myeloma Hub on Twitter shortly after the debate, voters largely agreed that anti-CD38 antibody-based therapies have demonstrated efficacy in patients with MM and should be routinely integrated into induction therapies (Figure 1).
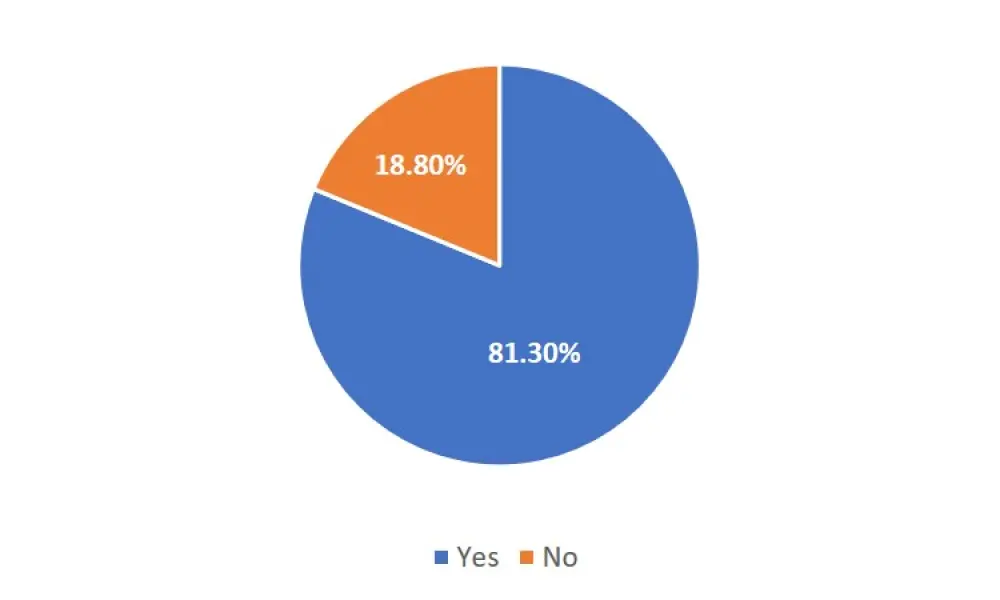
Figure 1. Results of the Multiple Myeloma Hub poll: Anti-CD38 antibody-based therapies have demonstrated efficacy in patients with MM, but should they be routinely integrated into induction therapies?
Follow these links for a further summary of the results of the CASSIOPEIA and GRIFFIN studies
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

