All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Monitoring immune dysfunction and reconstitution in multiple myeloma
At the 19th International Myeloma Society (IMS) Annual Meeting, Bruno Paiva discussed immune monitoring and its potential for predicting outcomes in patients with myeloma.1 Here, we summarize Dr Paiva’s discussion of the current research in immune reconstitution, as well as the challenges ahead.
Minimal residual disease status is not enough
Minimal residual disease (MRD) status is a powerful biomarker to distinguish two patient groups with different survival rates. However, when considering patients with undetectable or detectable MRD, predicting which patients will progress after 1 year compared with those that remain progression-free at successive intervals remains very challenging. MRD status alone is not sufficient to predict outcomes at this individual patient level.
Next-generation immune monitoring could help provide this key information. To do so, it will require standard operating procedures, new computational tools and large integrated datasets that include tumor, immunological, treatment and outcome data. With this, a better understanding of the mechanism of action of immunotherapies, clearly defined phenotypes of key immune cells, and a better understanding of bone marrow versus peripheral blood sampling (i.e., which sample to analyze and when) are needed.
Key immune cell phenotypes
Currently, a lack of understanding of key immune cell phenotypes precludes accurate monitoring of immunotherapy effects. Dr Paiva uses the monoclonal antibody daratumumab as an example. Whilst daratumumab is known to deplete CD38+ immune regulatory cells and promote T-cell expansion, information on the phenotypes of granulocytic myeloid-derived suppressor cells (G-MDSCs) and clonal T-cell expansion to effectively monitor the effect of the treatment has been lacking.
Using the established G-MDSC phenotype characterization, an analysis of bone marrow aspirates of healthy individuals compared with those with multiple myeloma reveals an overlap of 35–40% of granulocytes that includes neutrophils at various maturation stages and even common eosinophils. This common phenotype analysis of G-MDSC may therefore not be useful to evaluate treatment outcome. When the immunogenomic identification and characterization of G-MDSCs was subsequently carried out, the more immunosuppressive granulocytes were found to be mature neutrophils, characterized by the expression of CD11b+, CD13+, and CD16+. In the presence of a B‑cell maturation antigen (BCMA)–CD3 bispecific antibody, these cells showed the greatest potential to decrease T‑cell cytotoxicity and inhibit T‑cell proliferation, thus providing the possibility of more accurate monitoring of G-MDSCs, and ultimately treatment response.
A further example of immunogenomic characterization given by Dr Paiva was the study of clonal T‑cell phenotype. Most previous analyses have focused on markers associated with antigen-dependent differentiation and exhaustion. However, these markers provide no information as to whether a T cell is able to recognize and kill a myeloma cell. Immunogenomic characterization of clonal T cells showed that non-clonotypic T cells were enriched with CD27, whereas the majority of clonotypic T cells lacked this marker. Based on these observations, a CD27 T cell-negative to -positive ratio was found to be prognostic in patients with newly diagnosed multiple myeloma in two clinical trials using lenalidomide-based induction therapy.
Immune reconstitution in multiple myeloma
As well as the potential to better understand and monitor response to immunotherapies, the evaluation of immune reconstitution after transplant has the potential to shed light on survival outcomes. After generating large amounts of immunogenomic characterization data, three patient subgroups emerged, presenting with different patterns of immune reconstitution after autologous hematopoietic stem cell transplant and different survival. Interestingly, the presence of more terminally differentiated T cells was associated with poorer outcomes. Furthermore, a Spanish trial used multidimensional and computational flow cytometry to dissect the T‑cell compartment into >20 different subsets. Using all these data, a machine learning tool was used to develop immune signatures that were associated with progression-free survival and overall survival and showed no significant correlation with the patient’s MRD status.
The opportunity to individualize treatment
As this new era of immunogenomics and big data moves forward, there is exciting potential to individualize care through the development of computer algorithms in which patient data, including demographics, staging, genetics, and prior treatment, will be employed to assess the next appropriate treatment option based on predicted outcomes.
Dr Paiva showed a recent example from his group; a machine learning model based on tumor and immune biomarkers was used to predict MRD outcomes prior to treatment initiation in patients with newly diagnosed multiple myeloma (Figure 1). Using this machine learning model, MRD outcomes were accurately predicted in ~70% of patients. Of note, immune cell types as a predicting variable showed the highest coefficient of correlation with MRD outcomes.
Figure 1. Predicting MRD outcomes before treatment initiation*
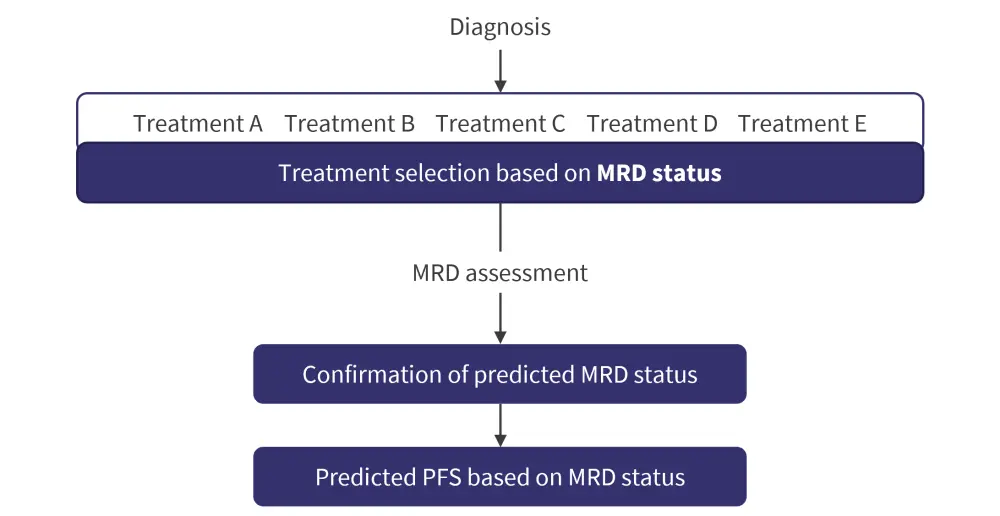
MRD, minimal residual disease; PFS, progression-free survival.
*Adapted from Pavia.1
This machine learning technology can also be applied to predict survival outcomes of severe infection. Employing data obtained by only two immune cell types alongside relevant patient data, this innovative approach predicted with great accuracy patient immunogenetic profile and survival after COVID-19 vaccination.
Conclusion
Monitoring immune dysfunction and reconstitution shows interesting potential to allow accurate predictions of disease progression and tailored immunotherapy for individual patients. Additionally, it can help detect loss of immunotherapeutic function of CAR T-cell therapies and predict risk of severe infection.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



