All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Incorporating PET imaging in a new risk stratification system for NDMM
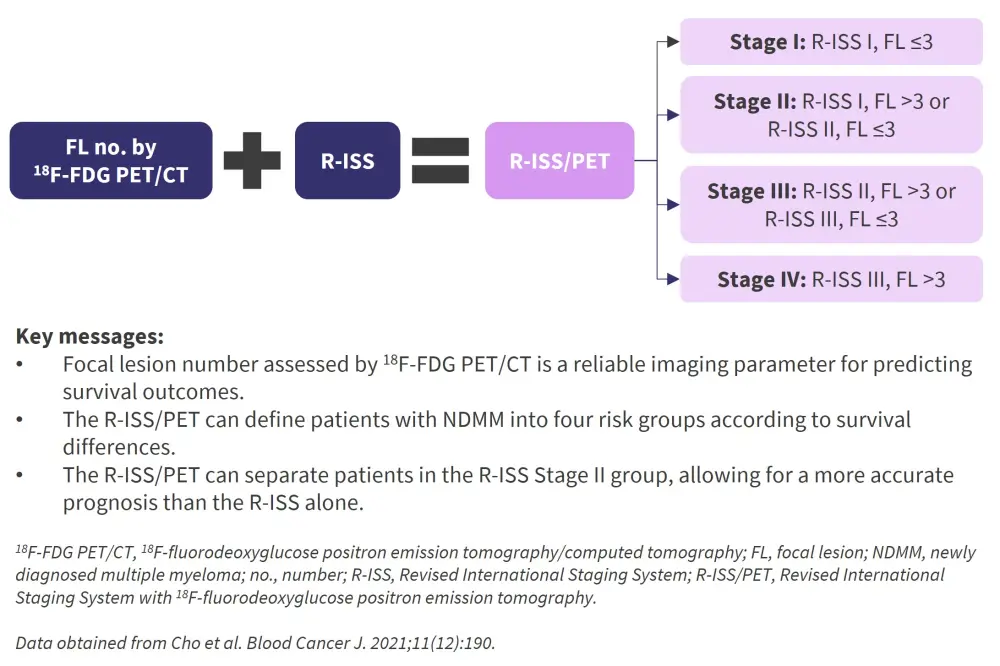
Patients with newly diagnosed multiple myeloma (NDMM) are risk stratified according to the Revised International Staging System (R-ISS) designed by the International Myeloma Working Group (IMWG).1 The R-ISS is based on parameters including the presence of beta-2 microglobulin, serum albumin levels, serum lactate dehydrogenase levels, and the presence of cytogenetic abnormalities. However, other high-risk factors such as the presence of multiple bone lesions and extramedullary disease have not been incorporated into the model. Recently, the IMWG recommend the use of imaging techniques, such as computed tomography (CT), magnetic resonance imaging, and 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT), to aid in the diagnosis of MM.1
Cho et al. along with the Korean Multiple Myeloma Working Party,1 recently designed a new risk stratification system that incorporates 18F-FDG PET/CT analysis with the R-ISS to effectively stratify patients with NDMM according to their survival outcomes. The development and validation of this new risk stratification model, the R-ISS/PET, was published in the Blood Cancer Journal and is summarized below.
Study design and patient characteristics
- The initial cohort consisted of 380 patients with NDMM who had 18F-FDG PET/CT analysis upon diagnosis across ten hospitals of the Korean Multiple Myeloma Working Party from September 2009 to March 2020.
- Patient characteristics can be seen in Table 1.
Table 1. Patient characteristics*
|
Auto-HSCT, autologous hematopoietic stem cell transplantation; CA, cytogenetic abnormality; ECOG PS, Eastern Cooperative Oncology Group performance status; iFISH, interphase fluorescent in situ hybridization; LDH, lactate dehydrogenase; PET/CT, positron emission tomography/computed tomography; R-ISS, Revised International Staging System. |
|
|
Characteristic, % (unless otherwise stated) |
n = 380 |
|---|---|
|
Median age (range), years |
66 (34–86) |
|
≥65 years |
54.5 |
|
Male/Female |
52.1/47.9 |
|
ECOG PS, % |
|
|
0–1 |
77.4 |
|
2–3 |
20.0 |
|
Unknown |
2.6 |
|
Increased LDH |
27.9 |
|
Albumin ≥3.5 g/dL |
61.6 |
|
Beta-2 microglobulin ≥ 5.5 mg/L |
36.8 |
|
CA by iFISH |
|
|
Standard risk |
81.3 |
|
High risk |
18.7 |
|
R-ISS |
|
|
I |
20.5 |
|
II |
60.5 |
|
II |
18.9 |
|
Extramedullary disease |
13.4 |
|
No of focal lesions on PET/CT |
|
|
≤3 |
47.6 |
|
>3 |
52.4 |
|
Frontline therapy |
|
|
Proteasome inhibitors |
80.8 |
|
Immunomodulatory agents |
49.5 |
|
Auto-HSCT |
34.5 |
- Regarding treatment response, 27.1% of patients had a complete response, 28.2% had a very good partial response, 29.7% had a partial response, 9.7% had stable disease, and 5.3% had progressive disease.
- The validation cohort consisted of 67 patients with NDMM from one hospital who had 18F-FDG PET/CT analysis upon diagnosis between June 2006 and February 2021.
- Baseline characteristics were similar to those in the original cohort.
- Median follow-up was 26 months (range, 0.1–153 months)
Results
Survival rates by focal lesions on 18F-FDG PET/CT evaluation or R-ISS
- Survival rates by the number of focal lesions on 18F-FDG PET/CT or R-ISS stage can be seen in Table 2.
- The 2-year overall survival (OS) and 2-year progression-free survival (PFS) rates differed significantly between patients with ≤3 focal lesions and those with >3 (p = 0.094 and p < 0.001, respectively).
- The 2-year OS and 2-year PFS rates differed significantly between the different R-ISS stages (both p < 0.001), with lower rates for higher stages.
Table 2. Survival rates by the number of focal lesions or R-ISS stage*
|
18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; FL, focal lesions; OS, overall survival; PFS, progression-free survival; R-ISS, Revised International Staging System. |
||
|
|
2-year PFS rate, % |
2-year OS rate, % |
|---|---|---|
|
Number of FL on 18F-FDG PET/CT |
|
|
|
≤3 FL |
78.0 |
84.2 |
|
>3 FL |
42.9 |
62.5 |
|
R-ISS stage |
|
|
|
Stage I |
71.8 |
95.3 |
|
Stage II |
53.2 |
82.9 |
|
Stage III |
26.8 |
61.2 |
Combining the number of focal lesions on 18F-FDG PET/CT with the R-ISS
A K-adaptive partitioning algorithm was undertaken to provide a statistically optimized combination of R-ISS with the number of focal lesions seen on 18F-FDG-PET/CT. Patients were defined into four groups, which successfully distinguished the patients with regard to 2-year PFS (p < 0.001) and 2-year OS (p < 0.001), as seen in Table 3. This was independent of transplant eligibility and treatment type.
Table 3. Survival rates by R-ISS/PET*
|
FL, focal lesions; OS, overall survival; PFS, progression-free survival; R-ISS/PET, Revised International Staging System with 18F-fluorodeoxyglucose positron emission tomography. |
||||
|
R-ISS/PET stage |
Combination |
% of patients |
2-year PFS |
2-year OS |
|---|---|---|---|---|
|
I |
R-ISS I with FL ≤3 |
8.2 |
84.1 |
96.7 |
|
II |
R-ISS I with FL >3 and R-ISS II with FL ≤3 |
41.1 |
64.7 |
89.8 |
|
III |
R-ISS II with FL >3 and R-ISS III with FL ≤3 |
42.6 |
40.8 |
74.7 |
|
IV |
R-ISS III with FL >3 |
8.2 |
17.1 |
50.3 |
The C-index values for OS and PFS were 0.668 (0.609–0.725) and 0.657 (0.615-0.698), respectively.
Multivariate Cox analysis (Table 4) also showed the R-ISS/PET was a significant factor in predicting long-term outcomes with regard to PFS and OS, with an increasing hazard ratio with each stage compared to Stage I.
Table 4. Multivariate analysis of factors affecting PFS and OS with the R-ISS/PET*
|
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EMD, extramedullary disease; HR, hazard ratio; OS, overall survival; PFS, progression free survival; R-ISS/PET, Revised International Staging System with 18F-fluorodeoxyglucose positron emission tomography. |
||||
|
Factor |
PFS |
OS |
||
|---|---|---|---|---|
|
HR (95% CI) |
p value |
HR (95% CI) |
p value |
|
|
Age, ≥65 years vs <65 years |
1.33 (0.98–1.80) |
0.063 |
1.56 (0.99–2.45) |
0.053 |
|
ECOG PS, 2–3 vs 0–1 |
1.01 (0.91–1.13) |
0.848 |
1.14 (1.01–1.28) |
0.034 |
|
EMD |
1.22 (0.80–1.84) |
0.353 |
1.68 (0.97–2.91) |
0.062 |
|
R-ISS/PET stage |
|
|
|
|
|
I |
1.00 |
|
1.00 |
|
|
II |
2.21 (1.00–4.90) |
0.050 |
2.50 (0.59–10.7) |
0.215 |
|
III |
4.57 (2.09–10.0) |
<0.001 |
5.11 (1.23–21.3) |
0.035 |
|
IV |
9.48 (3.88–12.2) |
<0.001 |
10.3 (2.24–47.0) |
0.003 |
Validation cohort
When assessed by the R-ISS stage, the external validation cohort showed similar 2-year PFS rates and 2-year OS rates to the initial cohort (Table 5). The classic stages showed a good degree of separation for survival outcomes (p = 0.268 for 2-year PFS and p = 0.037 for 2-year OS).
Table 5. Survival rates by R-ISS stage in the validation cohort*
|
OS, overall survival; PFS, progression-free survival; R-ISS, Revised International Staging System. |
||
|
R-ISS stage |
2-year PFS rate, % |
2-year OS rate, % |
|---|---|---|
|
I |
88.9 |
100 |
|
II |
60.4 |
80.3 |
|
III |
39.3 |
61.3 |
When the external validation cohort was assessed by the R-ISS/PET (Table 6), the stages also showed a significant separation for survival outcomes (p = 0.004 for 2-year PFS and p = 0.001 for 2-year OS).
Table 6. Survival rates by R-ISS/PET stage in the validation cohort*
|
OS, overall survival; PFS, progression-free survival; R-ISS/PET, Revised International Staging System with 18F-fluorodeoxyglucose positron emission tomography. |
|||
|
R-ISS/PET stage |
% of patients |
2-year PFS, % |
2-year OS, % |
|---|---|---|---|
|
I |
2.6 |
100 |
100 |
|
II |
27.6 |
74.5 |
89.9 |
|
III |
40.8 |
57.9 |
82.6 |
|
IV |
17.1 |
25.6 |
42.0 |
Conclusion
The number of focal lesions assessed by 18F-FDG PET/CT was demonstrated to be a reliable imaging parameter for predicting survival outcomes and therefore a feasible factor to be incorporated with the R-ISS. This new risk stratification model, the R-ISS/PET, was validated by an external cohort and was able to define patients with NDMM into four risk groups according to survival differences. The R-ISS/PET was able to separate patients in the R-ISS Stage II group (which accounted for ~60% of the population) and allows for a more accurate prognosis than the R-ISS.
This study is limited by the retrospective nature of the analysis as there was no standardization of the interpretation of the imaging analysis. Therefore, the results may be subjected to inter-hospital variations between the ten centers where the data were collected. Furthermore, false negatives could be possible for patients with extensive bone involvement but low expression of hexokinase-2 (the enzyme involved in the glycolysis of FDG in malignant cells). Therefore, a prospective study using newer high-risk fluorescent in situ hybridization markers such as t(14;20), gain(1q21), and del(1p32) may be required to further validate the R-ISS/PET model.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

