All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Immunotherapies and a future outlook on clinical trial designs
Recent immune therapy approvals including novel approaches, such as CAR T-cell therapy, bispecific antibodies, and antibody–drug conjugates, represent a significant advancement in the treatment landscape for multiple myeloma (MM).1 While response rates for these new therapies are often favorable, these therapeutic approaches highlight new safety and efficacy considerations, which in turn provide opportunities for innovative implementation into standard clinical trial designs.1
In response to this, Lancman et al.1 published a review in the American Journal of Hematology outlining areas of consideration for the incorporation of these new therapies into current clinical trial designs and what future trial designs may look like. Key areas include patient selection, combination therapies, and the use of current trial endpoints. We summarize the review below.
Patient selection
Future trial designs should be tailored to elderly and frail patients
Advances in the treatment landscape of MM result in many elderly patients surviving for several years after diagnosis. Thus, current trial designs require adaptation to fully include this patient subgroup. Based on frailty scores considerations, this patient population has commonly been excluded from MM trials, due to inferior overall survival and general increased risk of adverse events compared with other patient groups. Although comprehensive safety profiles of specific toxicities require further understanding, immune therapy now offers extremely low rates of organ toxicity, which could prove advantageous for patients of older age and with comorbidities. With regards to efficacy evaluation, it is also important to consider that a decline in T-cell function is often observed with older age and anti-MM therapy. Particularly, progression of MM results in overall reduced effector T-cell and NK-cell function, as well as decline of memory T-cell levels, all of which impact CAR T-cell therapy and bispecific antibody therapy. Based on these safety and efficacy observations, randomized trial designs should stratify patients based on age and Eastern Cooperative Oncology Group performance scores, while offering subgroup analyses data. Additionally, pilot studies tailored to patients of older age should include geriatric assessments, frailty scores, and patient-reported outcomes to help optimize patient selection. Check out our previous article on effective care in the elderly population published on the Multiple Myeloma Hub.
Considering renal function to inform pilot trial design
Currently, there is no evidence of significant renal clearance in novel immunotherapy trials; however, many clinical trials still include varying creatinine clearance cutoffs as part of their patient selection process. Of note, no trials involving immune therapies have shown renal toxicity that resulted from treatment and several more have highlighted similar response rates in patients with renal impairment compared to patients who had no impairment. Despite these data, patients with severe cases of renal deficiency or patients on hemodialysis remain unlikely to be put forward for larger trials due to expected overall worse prognosis and potential safety hurdles. To tackle this challenge, pilot trials should be developed and targeted towards this patient subpopulation to help expand access to these novel treatment options. In this context, it is important to distinguish between patients with rapidly declining renal function, who may benefit from “off-the-shelf” interventions such as bispecific antibodies, and those with stable renal output, who may in turn help assess glomerular filtration rate for lymphodepleting chemotherapy dosing within CAR T-cell immunotherapy.
Centralized assessment of patient cytogenetic risk may help optimize future randomized trial
Patients who present with high-risk cytogenetics at diagnosis often experience inferior outcomes compared with those considered at lower risk. Determining a patient’s cytogenetic risk status is currently hindered by several challenges, including:
- Varied testing technologies
- Lack of consensus on the definition of “high risk”
- Reporting of revised International Staging System stage
- Lack of data on clonal and allele burdens for specific abnormalities
- No clear evidence that recent therapies overcome high-risk status
The use of central labs for risk assessment and reporting of “high-risk” status evaluation should be of note in future randomized trials. Assessment of hazard ratios of high-risk against standard risk patients should also be considered. In contrast, targeted trials are becoming less practical, due to the small number of patients with MM presenting with high-risk cytogenetics in conjunction with an already rare malignancy.
Inclusion of extramedullary disease for comprehensive evaluation of efficacy and dosing
The inclusion of patients with extramedullary disease into early clinical trials would allow for the assessment of tissue penetration, pharmacokinetics and pharmacodynamics, and dosing issues associated with novel immune therapy treatments in MM.
Areas of consideration for combination therapies
In relation to the availability of multiple immune therapies, it is now also important to consider several factors, including:
- Whether patients can switch from one therapy to another
- Switching from one target to another within the same modality
- Effect on safety and efficacy based on the treatment used first
Other biological considerations include:
- Antigen expression at the time of progression
- T-cell fitness at the time of relapse
- Effects of lymphodepleting chemotherapy
Interestingly, preliminary findings support a general view that patients can benefit from more than one immune therapy provided they meet eligibility criteria, in particular cytopenias. Should evidence continue to support these findings, future immune therapy clinical trial design should incorporate the allowance of prior immune therapy exposure.
Figure 1. Key considerations for patient selection in future trial designs*
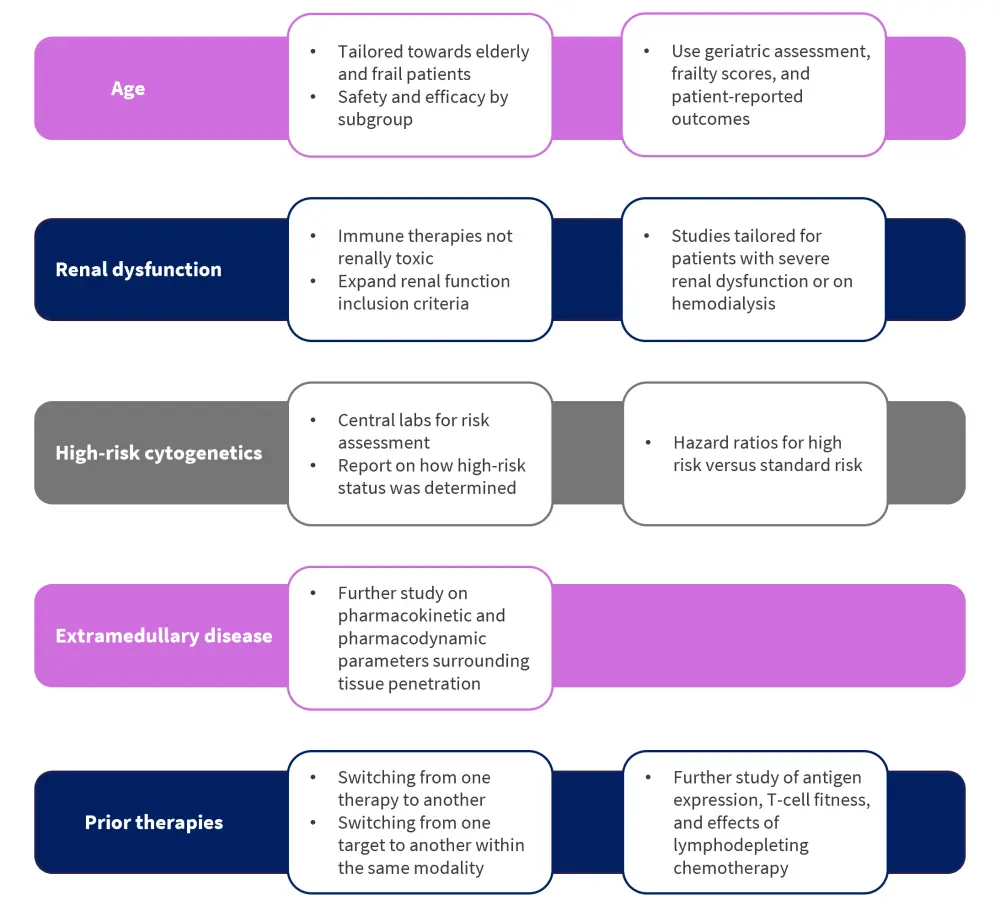
*Adapted from Lancman, et al.1
Optimizing dosing in early phase trials
New model-assisted trial designs show a more adaptable approach during phase I trials
Although current 3+3 designs widely used for phase I trials allow for ease of implementation, there is notable inefficiency in relation to determining a dose level that meets the required target toxicity level. This often leads to either overexposure above the maximum tolerated dose (MTD), or too little exposure that falls below the MTD. Instead, new model-assisted designs, such as the Bayesian Optimal Interval, provide a more adaptable framework and can target dose-limiting toxicity rates above or below the usual 25–33%. Superiority of the new model designs has been highlighted by the fact that they can select the true MTD at an increased rate, while allocating a higher percentage of patients at the maximum dose and fewer patients receiving suboptimal dosing. Furthermore, these newer designs require less statistical supervision. However, it should be noted that considering the higher doses to always be the most efficacious may be misleading, since some immune therapies tend to plateau in efficacy once the required immune activation has been achieved. In these specific cases, patients may be needlessly exposed to unnecessary toxicity levels. But recently, there has been a notable shift away from MTD and towards identifying the optimal biologic dose to provide a comprehensive view on toxicity, biological activity, and efficacy.
Phase II trials and the incorporation of large numbers of dose expansion cohorts
Determining the optimal dosing schedule remains a challenge for immune therapies. In particular, IgG- based therapies possess an expected half-life of 2–3 weeks, thus may require continuous dosing. Furthermore, bispecific antibodies have a time to first response of around 1 month and so present an opportunity for future trials to evaluate different maintenance schedules after initial induction.
Currently, several studies use large numbers of dose expansion cohorts, which allows for the informal evaluation of large numbers of dosing schema and can then be formally compared with the randomized phase II design. Multiple dosing schedules can therefore be simultaneously assessed and the optimal one selected on efficacy, toxicity, and quality of life. Another approach would be to include an initial induction period in study arms and different maintenance schedules afterwards. It should also be determined whether patients are to be treated until disease progression or until a predefined endpoint.
Recent therapy-associated toxicities and treatment discontinuations place an even greater emphasis on dose scheduling within MM. Future trial designs should therefore include rigorous continuous toxicity stopping rules and parameters for toxicity thresholds and boundaries. Continuous monitoring is also recommended.
Potential resistance mechanisms and unusual toxicities during combination therapy
Due to the heterogeneous immunological nature of the disease, the use of combination therapies has traditionally provided more favorable outcomes in patients with MM. However, immune therapy combinations still require a greater understanding of the mechanisms of resistance or non-response observed in monotherapy clinical settings. For example, loss or change in antigen expression in tumor cells may still account for resistance mechanisms in patients progressing through CAR T-cell therapy. In this context, gamma secretase inhibitors represent one option to counter this through increasing expression of B-cell maturation antigen on the surface of tumor cells and decreasing levels of soluble antigen. Moreover, the role of checkpoint inhibitors within the area of combination therapies remains unclear; initial results from studies suggest a positive relationship, but long-term follow-ups are still needed. Among combination therapy studies however, unusual and unexpected toxicities have also been reported, such as the unexpected increased mortality signals caused by immunomodulatory imide drugs in late phase combination trials with an anti-PD1 checkpoint inhibitor. As mentioned above, continuous monitoring is recommended for all future phase II combination trials.
Figure 2. Key considerations for optimizing dosing in early phase trials*
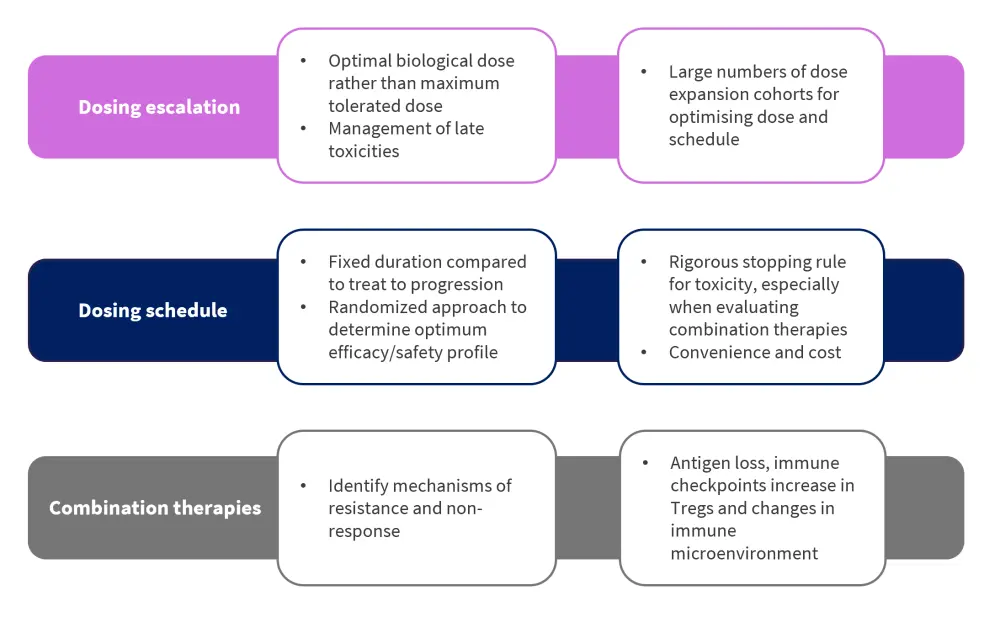
Treg, regulatory T cell.
*Adapted from Lancman, et al.1
Refinement of current trial endpoints
Safety
Several specific safety signals require further evaluation, including:
- Immune-related toxicities
- Delayed toxicities
- Hypogammaglobulinemia/infection
Prediction and prevention of immune-related toxicities
- Cytokine release syndrome (CRS)
- Immune effector cell-associated neurotoxicity syndrome
- Macrophage activation syndrome
While management strategies are in place for most immune-related toxicities, algorithms to help predict or prevent these conditions are still needed. For example, mitigation procedures for CRS are well understood, however, CRS itself remains unpredictable in terms of onset and severity, especially in relation to CAR T-cell therapy. Accurate predictive models or prophylactic steroids would therefore be a valuable endpoint for future trial designs, expanding access while reducing costs and burden on the healthcare system.
Long-term follow-ups for delayed toxicities
Delayed toxicities in relation to immune therapies remain poorly understood. The CARTITUDE-1 study (NCT03548207) reported 12% of patients with non-immune effector cell-associated neurotoxicity syndrome neurotoxicity, with one case being identified 101 days post-treatment, suggesting a potential monitoring period of neurotoxicities for up to 1 year. As the duration of available follow-up for many of these therapies is generally short, the full impact and scope of these developing toxicities must be closely monitored.
Understanding immune deficits for prophylactic prevention of infection
Infection is one of the leading causes of morbidity and mortality among patients diagnosed with MM. Current prophylactic measures vary widely across treatments and clinics, but normally include a combination of antibiotics, antivirals, antifungals, growth factors, and immunoglobulin replacement therapy. However, further study is needed to understand the immune deficiencies associated with each type of treatment and to help inform a more efficient and streamlined approach for all patients.
Adapted progression-free survival to allow for solitary lesions
Progression-free survival (PFS) remains a key outcome measure in the process of drug approvals, however, it has been noted that immune therapies may cause temporary inflammation at tumor sites, often mistakenly interpreted as disease progression. Similarly, there have been reported cases of new solitary plasmacytoma which were successfully controlled with subsequent irradiation. Whether the combination of immune therapy and irradiation provides a tumor-shrinking effect remains yet to be fully understood; however, if consistent, then a modified PFS endpoint that accounts for irradiated lesions would better capture the trial clinical outcome. Duration of therapy and time to next treatment may also provide further indications not analyzed by PFS.
Overall survival
Although overall survival is not normally a primary endpoint in MM trials, it should be evaluated in all randomized phase III trials. However, although median overall survival remains generally high in patients treated with CAR T-cell therapy, early mortality signals compared with standard therapies have been observed, which would be unfavorable in earlier lines of treatment. To mitigate this, future trials should report all deaths by immediate cause and by disease response status.
Treatment comparison by minimal residual disease assessment
The assessment of minimal residual disease has become increasingly popular in MM trials. Analysis by next-generation sequencing is now able to quantify the depth of response in addition to whole body imaging, which helps comparison between treatments. In contrast, the use of minimal residual disease testing is currently centered around prognosis rather than prediction, and therefore, may require further optimization. Should MRD assessment be expanded to act as an endpoint, high sensitivity assays would be required, and assessment should be sustained for at least 1 year in conjunction with full body imaging.
Half-life of immune therapies and use of mass spectrometry to determine depth and time to response
To determine the depth of response and time to response, two factors must be considered. The first is the recycling and subsequent increase in half-life immunoglobulin G caused by the neonatal Fc receptor, which in turn can affect the accurate determination and coding of response. The second factor is the use of mass spectrometry to distinguish between drug and disease in patients with IgG-kappa MM treated with immunoglobulin G-kappa-based antibody therapies.
Underutilized role of quality of life as a trial endpoint
Currently, quality of life remains an underutilized endpoint in clinical trial designs. However, quality of life assessment can be key in determining patient wellbeing, treatment burden and predict healthcare utilization. Patient quality of life may vary substantially over time according to the type of therapy used but has the potential to play a crucial role in treatment selection.
Translational research to identify new phenotypes, immune microenvironment changes and levels of response in patients
Both current and future clinical trials provide the opportunity for translational research to refine patient selection, dosing, and combination therapy regimens. There are ongoing studies aiming to better identify phenotypes of myeloma cells, T cells, and other immune cells at several stages of treatment, as well as attempting to define key changes in the immune microenvironment during relapse. Further profiling of responders versus non-responders in MM immune therapy clinical trials will guide opportunities for the best course of treatment, as well as the identification of immunosuppressive cell populations for targeted combination therapies.
Figure 3. Key considerations in the refinement of current clinical trial endpoints*
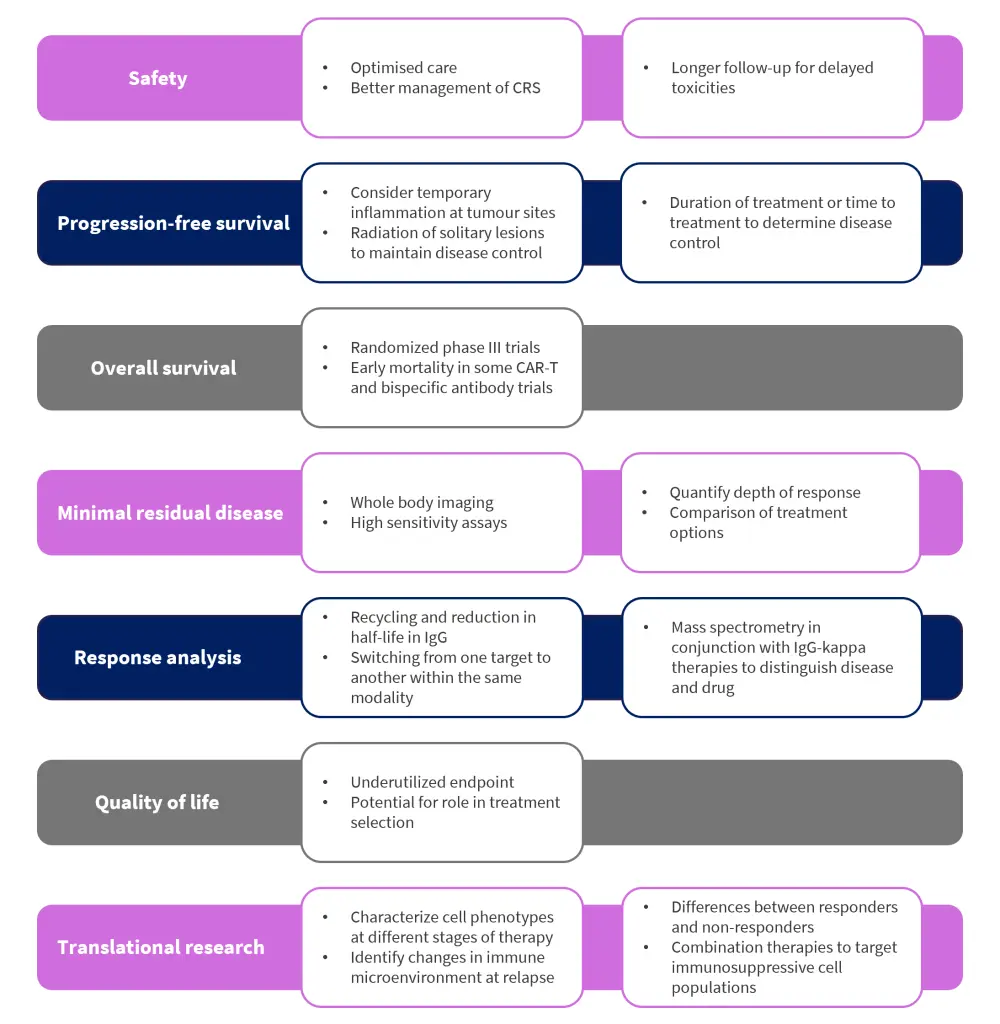
CAR, chimeric antigen receptor; CRS, cytokine release syndrome; IgG, immunoglobulin G.
*Adapted from Lancman, et al.1
Conclusion
It is encouraging to observe the development of several MM immunotherapies, which have either already received approval or are being investigated within late-stage clinical trials. The expansion of these novel approaches has proven relevant efficacy, especially in heavily relapsed patients with MM, and may soon shift treatment paradigms in the clinical setting. Future clinical trial designs should consider adaptive measures to accommodate their unique pharmacokinetic and pharmacodynamic properties, with the aim of optimizing patient selection dosing schema, and refining current trial endpoints to better leverage the potential of these new therapies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

