All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
IKEMA trial: Isa-Kd versus Kd for RRMM – final overall survival analysis
Do you know... The overall survival (OS) analysis of the IKEMA trial was planned 3 years post final progression-free survival analysis and showed significant OS improvements in the Isa-Kd arm versus the Kd arm. What was the median OS after 3 years in the Isa-Kd arm?
Isatuximab is an IgG1 monoclonal antibody which targets CD38 on myeloma cells. Therapeutic effects include induced apoptosis, immunomodulation, and ectoenzyme inhibition.1 The combination of isatuximab, carfilzomib, and dexamethasone (isa-Kd) was approved by both the U.S. Food and Drug Administration and the European Commission in 2021 for the treatment of patients diagnosed with relapsed/refractory multiple myeloma who have received 1–3 lines of prior therapy.
Approval was based on results obtained from the phase III IKEMA trial (NCT03275285) investigating isa-Kd versus carfilzomib and dexamethasone (Kd).
During the 20th International Myeloma Society Annual Congress, Moreau presented the final overall survival (OS) analysis from the IKEMA trial. Here, we summarize the key points from the presentation. For more information on the IKEMA trial, see the dedicated page on the Multiple Myeloma Hub.
Study design1
- The trial included a total of 302 patients who were randomized 3:2 to either isa-Kd or Kd alone.
- The full study design and eligibility criteria were previously reported on the Multiple Myeloma Hub.
- The primary endpoint of the trial was progression-free survival (PFS).
- The baseline patient characteristics for both treatment arms were well balanced (Table 1).
Table 1. Baseline patient characteristics*
|
IMiD, immunomodulatory agent; ISS, International Staging System; isa-Kd, isatuximab, carfilzomib, and dexamethasone; Kd, |
||
|
Characteristic, % (unless otherwise stated) |
Isa-Kd (n = 179) |
Kd (n = 123) |
|---|---|---|
|
Median age, years |
65.0 |
63.0 |
|
ISS stage |
||
|
I |
49.7 |
57.7 |
|
II |
35.2 |
25.2 |
|
III |
14.5 |
16.3 |
|
Cytogenetic risk |
||
|
High |
23.5 |
25.2 |
|
Standard |
63.7 |
63.4 |
|
Missing |
12.8 |
11.4 |
|
Number of prior lines of therapy |
||
|
1 |
44.1 |
44.7 |
|
2 |
35.8 |
29.3 |
|
3 |
18.4 |
24.4 |
|
Prior PI |
92.7 |
85.4 |
|
Prior IMiD |
76.0 |
81.3 |
|
Patients refractory to |
||
|
IMiD |
43.6 |
47.2 |
|
Lenalidomide |
31.8 |
34.1 |
|
PI |
31.3 |
35.8 |
|
Last regimen |
49.7 |
59.3 |
Results
- The final PFS and second progression-free survival analysis showed significant improvement with Isa-Kd versus Kd treatment (Figure 1).
Figure 1. Final PFS and PFS2 analysis for patients treated with isa-Kd and Kd*
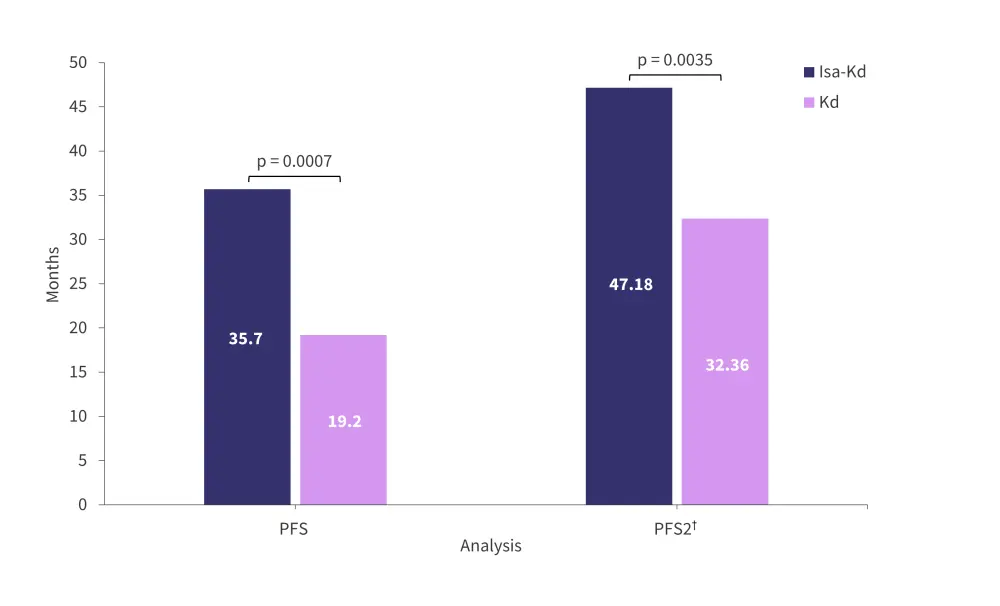
Isa-Kd, isatuximab, carfilzomib, and dexamethasone; Kd, carfilzomib and dexamethasone; PFS, progression-free survival; PFS2, second progression-free survival.
†Progression-free survival is defined as the time from randomization to disease progression or subsequent therapy/death.
*Adapted from Moreau.1
- A consistent treatment effect was observed across most subgroup comparisons.
- PFS benefit was maintained after the first subsequent therapy.
- Treatment duration was 1.5-times longer in the isa-Kd arm at 94 weeks versus 61.9 weeks in the Kd arm
- Both treatment arms had a similar relative carfilzomib dose intensity
- OS analysis was planned at 3 years post primary PFS analysis at a median follow-up duration of 56.61 months.
- Median OS was not reached in the isa-Kd arm versus 50.6 months in the Kd arm (p = 0.1836).
- Extrapolated median OS for the isa-Kd arm was 63 months.
- This corresponded to an estimated 1-year difference in median OS compared to the Kd arm.
- At the time of OS analysis, more patients remained on isa-Kd treatment (23.5%) versus Kd treatment (5.7%).
- Fewer patients discontinued due to disease progression in the isa-Kd arm (45.3%) compared with the Kd arm (53.7%).
- Time to next treatment for patients in the isa-Kd arm was 43.99 months versus 25 months for patients in the Kd arm (p = 0.0002).
Safety
- The safety profile was similar to the OS analysis at the final PFS analysis.
- The safety profile was also consistent with previous analyses.
- There was a similar incidence of cardiac failure in both arms, with 4.5% of patients on isa-Kd treatment experiencing a Grade ≥3 cardiac event versus 4.1% of patients treated with Kd.
- The proportion of treatment-emergent adverse events that were fatal or led to treatment discontinuation was similar between treatment arms.
- This was despite the extra 30-week treatment period for the isa-Kd group.
- The percentage of carfilzomib-associated events was also comparable between arms, despite the longer treatment period of isa-Kd.
Conclusion
Patients treated with isa-Kd benefited from a more favorable OS compared with Kd treatment. This represents the longest OS achieved in a phase III lenalidomide-free trial in the relapsed setting. The isa-Kd treatment arm also experienced a more favorable time to next treatment and second progression-free survival compared with the Kd arm. Moreover, the benefit of isatuximab was sustained through subsequent lines of therapy. Overall, the final analysis of the trial supports the use of isa-Kd as the standard of care option for patients diagnosed with relapsed/refractory multiple myeloma.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

