All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Iberdomide and dexamethasone with bortezomib or daratumumab for relapsed and refractory MM
Iberdomide (Iber) is a cereblon E3 ligase modulator. When it binds to cereblon, it causes increased degradation of proteins such as Ikaros and Aiolos, which are involved in the development of B cells and CD4+ T cells. Iberdomide has been shown to be active even in multiple myeloma (MM) cells that are resistant to lenalidomide and pomalidomide.
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Niels Van de Donk spoke about the results of the phase I/II trial CC-220-MM-001 (NCT02773030), which investigated bortezomib (Bort) or daratumumab (Dara) combined with iberdomide + dexamethasone (Dex).1
The Multiple Myeloma Hub previously reported on phase I results of cohorts A and B, which compared iberdomide single-agent with iberdomide + dexamethasone, and this article can be found here.
Study design
Eligibility criteria
- Diagnosed with relapsed and refractory MM
- Treated with ≥ 2 previous regimens, including lenalidomide/pomalidomide and a proteasome inhibitor
- Disease progression on or within 60 days of the last antimyeloma treatment
Key endpoints
- Primary: Determine the maximum tolerated dose/recommended phase II dose and efficacy
- Secondary: Safety
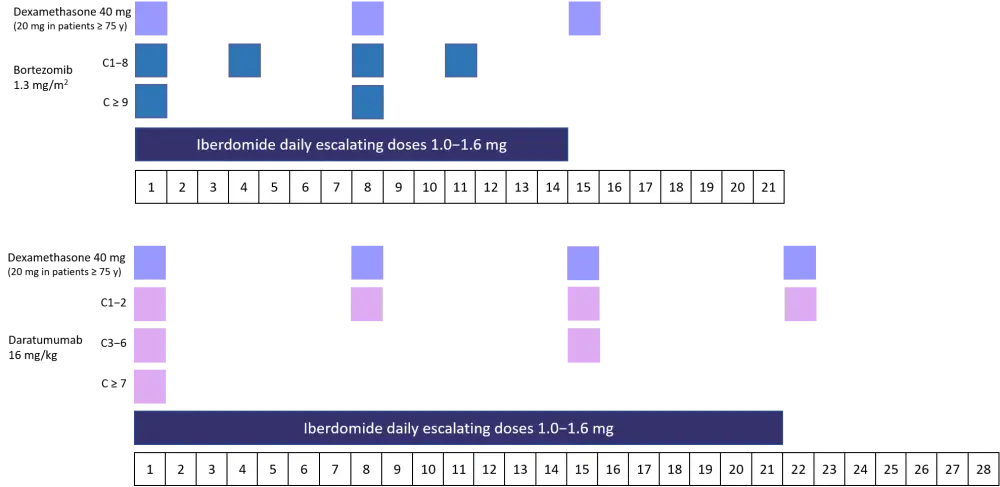
The dosing schedule for the two cohorts is shown in Figure 1.
Figure 1. Dosing schedule for bortezomib and daratumumab triplet cohorts2
Patient characteristics
The median age was similar between the two cohorts at 66 and 63 years for daratumumab and bortezomib, respectively. As both cohorts were > 7 years since diagnosis, these patients were advanced in the course of their disease. The majority of patients had an Eastern Cooperative Oncology Group performance score (ECOG PS) of 1 and an International Prognostic Scoring System of Stage 1 at the time of entry to the study (Table 1).
The median number of therapies previously received was slightly lower in the daratumumab cohort at 4 compared with 6 for the bortezomib group. Most patients were refractory to immunomodulatory drugs such as pomalidomide, and almost two-thirds were refractory to proteasome inhibitors. In the daratumumab arm, 55.6% of patients included were already refractory to daratumumab and 39.1% of patients in the bortezomib arm were refractory to this agent. The percentage of triple-class refractory patients was 48.1% in the daratumumab triplet and slightly lower at 39.1% in the bortezomib triplet group.
Table 1. Patient characteristics1
|
ASCT, autologous stem cell transplantation; Dara, daratumumab; Dex, dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance score; Iber, iberdomide; IMiD, immunomodulatory drugs; IPSS, International Prognostic Scoring System; PI, proteasome inhibitor |
||
|
Characteristic |
Iber + Dara + Dex (n = 27) |
Iber + Bort + Dex (n = 23) |
|---|---|---|
|
Age, median (range) years |
66 (40−77) |
63 (47−81) |
|
Male (%) |
55.6 |
73.9 |
|
Time since initial diagnosis, median (range), years |
8.0 (1.1−19.1) |
7.1 (3.0−16.0) |
|
ECOG PS (%) |
||
|
0 |
40.7 |
34.8 |
|
1 |
55.6 |
60.9 |
|
2 |
3.7 |
4.3 |
|
IPSS at study entry (%) |
||
|
Stage I |
51.9 |
56.5 |
|
Stage II |
29.6 |
39.1 |
|
Stage III |
14.8 |
4.3 |
|
Presence of extramedullary plasmacytoma (%) |
22.2 |
17.4 |
|
Prior therapies, median (range), n |
4 (2−12) |
6 (1−14) |
|
ASCT (%) |
88.9 |
87.0 |
|
IMiD-refractory (%) |
96.3 |
78.3 |
|
Pomalidomide |
77.8 |
52.2 |
|
PI-refractory (%) |
77.8 |
65.2 |
|
Bortezomib |
40.7 |
39.1 |
|
Carfilzomib |
59.3 |
34.8 |
|
Ixazomib |
18.5 |
17.4 |
|
Anti-CD83 mAb-refractory (%) |
55.6 |
78.3 |
|
Daratumumab |
55.6 |
73.9 |
|
Isatuximab |
0 |
4.3 |
|
Triple-class refractory (%) |
48.1 |
39.1 |
Key points
At the time of data cutoff, almost half of patients were still on treatment. By the cutoff date, patients had received a median number of cycles:
- Daratumumab triplet: 4 (range 1−18)
- Bortezomib triplet: 6 (range 1−22)
Discontinuations were most commonly due to disease progression (37% in the daratumumab cohort and 30.4% in the bortezomib cohort) rather than adverse events (two patients in the bortezomib cohort; one case of syncope thought to be treatment-related, and one unrelated case of pelvic pain). No deaths were recorded in this study. Only two incidences of dose-limiting toxicity were recorded, both in the bortezomib group. Both were Grade IV thrombocytopenia, with one case occurring at 1.1 mg/day and the other at 1.6 mg/day of iberdomide.
Additional patients were enrolled at various dose levels to further analyze safety and efficacy. Currently, these patients are being treated at 1.6 mg/day of iberdomide, and the recommended phase II dose should be established soon.
Adverse events in the daratumumab group were mostly hematologic, with neutropenia being the most common. In the bortezomib group, neutropenia and thrombocytopenia were equally frequent, although thrombocytopenia cases were more likely to be of Grade IV. Overall, there were few Grade III or IV adverse events, and therefore the triplets were well tolerated (Table 2).
Table 2. Safety profile of the two treatment regimens1
|
TEAEs, treatment-emergent adverse events; Dara, daratumumab; Dex, dexamethasone; Iber, iberdomide. *Includes neutropenic sepsis. |
||||||
|
TEAEs of interest, n (%) |
Iber + Dara + Dex (n = 27) |
Iber + Bort + Dex (n = 23) |
||||
|---|---|---|---|---|---|---|
|
All grades |
Grade III |
Grade IV |
All grades |
Grade III |
Grade IV |
|
|
Hematologic TEAEs |
||||||
|
Neutropenia |
19 (70.4) |
4 (14.8) |
14 (51.9) |
8 (34.8) |
5 (21.7) |
1 (4.3) |
|
Febrile neutropenia* |
1 (3.7) |
0 |
1 (3.7) |
0 |
0 |
0 |
|
Thrombocytopenia |
11 (40.7) |
3 (11.1) |
1 (3.7) |
8 (34.8) |
1 (4.3) |
5 (21.7) |
|
Anemia |
10 (37.0) |
7 (25.9) |
1 (3.7) |
5 (21.7) |
3 (13.0) |
0 |
|
Non-hematologic TEAEs |
||||||
|
Peripheral neuropathy |
2 (7.4) |
0 |
0 |
7 (30.4) |
0 |
0 |
|
Fatigue |
9 (33.3) |
0 |
0 |
6 (26.1) |
0 |
0 |
|
Diarrhea |
6 (22.2) |
1 (3.7) |
0 |
7 (30.4) |
1 (4.3) |
0 |
|
Decreased appetite |
— |
— |
— |
7 (30.4) |
0 |
0 |
|
Constipation |
6 (22.2) |
0 |
0 |
5 (21.7) |
0 |
0 |
|
Rash |
3 (11.1) |
0 |
0 |
6 (26.1) |
1 (4.3) |
0 |
|
Myalgia |
0 |
0 |
0 |
5 (21.7) |
0 |
0 |
|
Infusion-related reaction |
1 (3.7) |
0 |
0 |
— |
— |
— |
|
Insomnia |
— |
— |
— |
5 (21.7) |
0 |
0 |
|
Pruritis |
— |
— |
— |
5 (21.7) |
0 |
0 |
|
Infections |
21 (77.8) |
3 (11.1) |
2 (7.4) |
14 (60.9) |
3 (13.0) |
0 |
|
Upper respiratory tract infection |
10 (37.0) |
0 |
0 |
7 (30.4) |
2 (8.7) |
0 |
Immune profiling data generated from bone marrow and blood samples showed no notable changes in the pharmacodynamic effect of iberdomide + dexamethasone due to the addition of daratumumab or bortezomib. While daratumumab reduced the number of NK cells, iberdomide promoted proliferation of both NK cells and T cells and reduced the absolute B-cell count.
In terms of efficacy, in the daratumumab triplet cohort:
- The overall response rate was 42.3%
- The clinical benefit rate (CBR; calculated by addition of stringent complete response, complete response, very good partial response, partial response and minimal response) was 50%
- The disease control rate (DCR; CBR + stable disease) was 88.5%
- The median time to response was 4.1 weeks (range, 4.0−12.0 weeks)
In the bortezomib triplet cohort:
- The overall response rate was 60.9%
- CBR was 69.9%
- DCR was 87.0%
- A similar median time to response of 3.6 weeks (range, 3.0−13.1 weeks) was recorded
Conclusion
Iberdomide triplet combinations showed a favorable activity and safety profile in heavily pretreated patients with relapsed and refractory MM. Identification of the recommended phase II dose is still ongoing, and iberdomide is currently being tested at 1.6 mg/day in both triplet groups. The immune profiling results show that the immune stimulatory effect of iberdomide remained when used in combination, and is not significantly impacted by the addition of daratumumab or bortezomib. Together these results show that iberdomide combinations are effective in patients with relapsed and refractory MM and warrant testing in phase III studies, which are currently being planned. The ongoing trials involving iberdomide are listed in Table 3.
Table 3. Ongoing clinical trials involving iberdomide3
|
ASCT, autologous stem cell transplantation; Dex, dexamethasone; Iber, iberdomide; MM, multiple myeloma. |
||||
|
NCT number |
Title |
Agents tested |
Phase |
Status |
|---|---|---|---|---|
|
Iberdomide (Cc220) maintenance after ASCT in newly diagnosed MM patients |
Iber |
II |
Not yet recruiting |
|
|
Iberdomide combined with low-dose cyclophosphamide and dexamethasone (ICON) |
Iber + Dex + low-dose cyclophosphamide |
II |
Recruiting |
|
|
Iberdomide alone or in combination with dexamethasone for the treatment of intermediate- or high-risk smoldering multiple myeloma |
Iber + Dex |
II |
Not yet recruiting |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

