All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Exploring the optimal dose for lenalidomide maintenance after auto-SCT
The standard of care for newly diagnosed patients with multiple myeloma (MM) eligible for transplant is high-dose chemotherapy induction, followed by autologous hematopoietic stem cell transplantation (auto-HSCT) and lenalidomide (Len) maintenance therapy (MT) to prolong remission. Due to frequent and severe side effects, Len MT dosing is often reduced, yet the dose-response relationship of Len MT has not been fully described.
Roland Fenk and colleagues from Germany have recently published the results from their multicenter phase III trial (NCT00891384), in which patients were randomized to receive high- or low-dose Len MT. The study, published in Cancer Research, aimed to assess the efficacy and tolerability of two extreme Len doses, and share experience-based recommendations for the optimal MT with Len.1
Recommended guidelines to optimize Len maintenance therapy1 |
|
Study design
Patients were 18–75 years old, diagnosed with MM, and treated with ≤ 6 cycles of induction (not with Len), up to 2 cycles for mobilization, and auto-HSCT (between 90–120 days) before trial entry. Patient randomization was stratified by International Staging System (ISS) stage, age at diagnosis, and response after high-dose chemotherapy. All enrolled patients received Len at a dose of 25 mg/day for 21 days, every 28 days for 6 months (Len6), and then continued with their assigned dose: the high-dose arm moved onto Len MT at a dose of 25 mg/day, and the low-dose arm began Len MT at 5 mg/day. Maintenance therapy was prescribed until disease progression or limiting toxicity (Figure 1).
The primary outcome of the study was progression-free survival (PFS), and secondary endpoints included overall survival (OS), response rates, adverse events (AE), and incidence of secondary primary malignancies.
Figure 1. Study design1
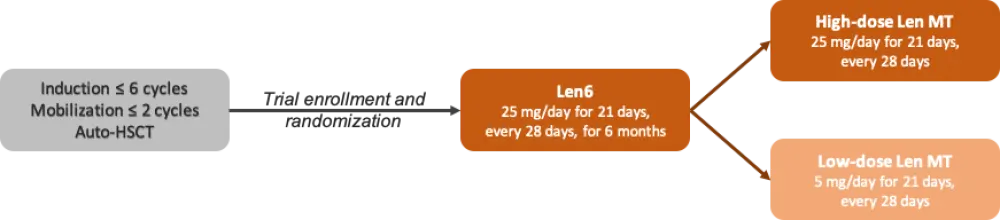
auto, autologous; HSCT, hematopoietic stem cell transplant; Len, lenalidomide; MT, maintenance therapy.
Results
- The study enrolled 194 patients.
- 188 patients were randomized to high dose Len MT (n = 94) or low dose Len MT (n = 94).
- There was no significant difference in patient characteristics between arms (Table 1).
Table 1. Patient characteristics
|
|
|
High-dose arm (n = 94) |
Low-dose arm (n = 94) |
|
auto, autologous; CR, complete response; FISH, fluorescence in situ hybridization; HDCT, high-dose chemotherapy; HSCT, autologous stem cell transplantation; ISS, International Staging System; LC, light chain; MR/SD, minimal response/stable disease; PR, partial response; VGPR, very good partial response. * Defined as: amp(1q), t(4;14), t(14; 16), del(17p). |
|||
|
Age, years |
median (range) |
58 (33–71) |
58 (30–72) |
|
|
≤ 65, % |
77 |
77 |
|
Subtype, % |
IgG |
60 |
58 |
|
|
IgA |
20 |
17 |
|
|
LC |
21 |
23 |
|
|
Other |
0 |
2 |
|
ISS-stage, % |
I |
54 |
57 |
|
|
II |
27 |
20 |
|
|
II |
19 |
23 |
|
FISH cytogenetic risk, % |
Standard |
27 |
22 |
|
|
High* |
16 |
15 |
|
|
Unknown |
57 |
63 |
|
Chronic kidney disease stage, % |
1 and 2 |
76 |
72 |
|
|
3 |
8 |
14 |
|
|
4 |
5 |
5 |
|
|
5 (dialysis) |
11 |
9 |
|
Induction therapy with bortezomib, % |
|
81 |
85 |
|
|
Median no. cycles (range) |
3 (1–6) |
3 (1–6) |
|
Response after HDCT, % |
CR |
16 |
28 |
|
|
VGPR |
49 |
37 |
|
|
PR |
29 |
32 |
|
|
MR/SD |
6 |
3 |
|
Time from diagnosis to baseline, months |
Median (range) |
10 (7–19) |
11 (5–21) |
- After a median follow-up of 46.7 months, median PFS in the high-dose arm was 44.8 months vs 33.0 months in the low-dose arm (HR, 0.65; 95% CI, 0.44–0.97; p = 0.032).
- Longer PFS was seen in patients < 66 years, with revised ISS stages II and III at diagnosis, and with ≤ PR after auto-HSCT.
- The median duration of treatment was significantly longer in the high-dose Len MT arm (26.8 vs 22.9 months, p = 0.01).
- Median OS was not reached in either arm.
- Stringent complete response (sCR) rates increased from 6% at randomization to 23% after the initial 6 months with Len in the high-dose arm, and from 9% to 19% in the low-dose arm. Improvement in the remission status in the following years was less frequent at each time point but was still observed until 3.5 years after randomization (Table 2).
Table 2. Response rates in treatment arms over the study course. All values are % unless otherwise specified1
|
Arm |
High-dose arm |
Low-dose arm |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
CR, complete response; Len, lenalidomide; Len6, Len 25 mg/day for 21 days, every 28 days for 6 months; MR/SD, minimal response/stable disease; MT, maintenance; ORR, overall response rate; rand., randomization; sCR, stringent complete response; VGPR, very good partial response. |
||||||||||
|
Timepoint |
Rand. |
Len6 |
Len MT |
Rand. |
Len6 |
Len MT |
||||
|
Time, years |
0 |
0.5 |
1 |
2 |
3 |
0 |
0.5 |
1 |
2 |
3 |
|
Response |
Patients, % |
|||||||||
|
sCR |
6 |
23 |
30 |
21 |
15 |
9 |
19 |
18 |
15 |
13 |
|
CR |
10 |
12 |
13 |
7 |
4 |
19 |
21 |
17 |
13 |
6 |
|
VGPR |
49 |
35 |
21 |
13 |
6 |
37 |
27 |
12 |
7 |
3 |
|
PR |
29 |
21 |
14 |
11 |
5 |
32 |
24 |
14 |
7 |
2 |
|
MR/SD |
6 |
4 |
4 |
3 |
2 |
3 |
2 |
1 |
1 |
0 |
|
Still receiving Len |
– |
– |
72 |
44 |
24 |
– |
– |
52 |
34 |
15 |
- The median exposure in the high-dose arm until disease progression was 14.5 mg/day, following various dose adjustments.
- Dose reductions needed throughout the study were distributed as follows (% over the total dose reductions):
- Len6: 53%
- High-dose Len MT: 39%, although it did not translate into higher discontinuations
- Low-dose Len MT: 8%
- 55.5% of dose reductions were due to neutropenia.
- There were more Grade ≥ 3 AEs in the high-dose arm (87.5%) than the low-dose arm (64.6%).
- Grade ≥ 3 neutropenia and thrombocytopenia were the main adverse events during Len6 (39.0% high-dose arm, 8.9% low-dose arm).
- Grade ≥ 3 neutropenia incidence reduced over the 1–3 years of Len MT in the high-dose arm (34.6% in Year 1, 24.3% in Year 2, and 12.8% in Year 3), but remained constant in the low-dose arm (9%).
- Grade ≥ 3 infections were experienced by 12% of patients during Len6 and reduced during MT (from 12.3% to 7.7% after 3 years, in the high-dose arm).
Conclusions
The research team concluded that their data demonstrate an enhanced PFS (of approximately 1 year) with a high-dose Len MT, and that there is a trend towards improved OS, but that this comes with increased hematological toxicities. The investigators also highlighted that although dose reductions were frequent in the high-dose arm, AEs resulting in discontinuation of treatment were similar in both groups, and the high-dose arm had a significantly longer duration of treatment. They state that the maximum tolerated dose varies and should be up-/down-titrated on a case by case basis. The recommended guidelines to optimize Len maintenance therapy are given at the start of this article, see above.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

