All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
European Medicines Agency recommends marketing authorization for elranatamab
On October 13, 2023, the Committee for Medicinal Products for Human Use (CHMP), part of the European Medicines Agency (EMA), announced the recommended marketing authorization for elranatamab. The United States Food and Drug Administration (FDA) previously granted approval under the accelerated approval program in August 2023.
Elranatamab is a novel B-cell maturation antigen CD3-directed bispecific antibody intended for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have received ≥3 prior lines of therapy and have experienced disease progression during the most recent treatment. Elranatamab binds to BCMA on myeloma cells and CD3 on T-cells, leading to T-cell activation and myeloma cell death.
The recommendation was based on the results from the phase II MagnetisMM-3 trial (NCT04649359), which enrolled 123 patients.
- The objective response rate was 61%.
- There was a 72% probability of maintaining response at 15 months.
- After 24 weeks of weekly infusions, an every-other-week dosing schedule was established.
- Of the patients who switched to the every-other-week dosing schedule (n = 50), 80% maintained or improved their response and 38% experienced a complete response.
- The ten most common adverse events are shown in Figure 1.
Figure 1. Most common adverse events*
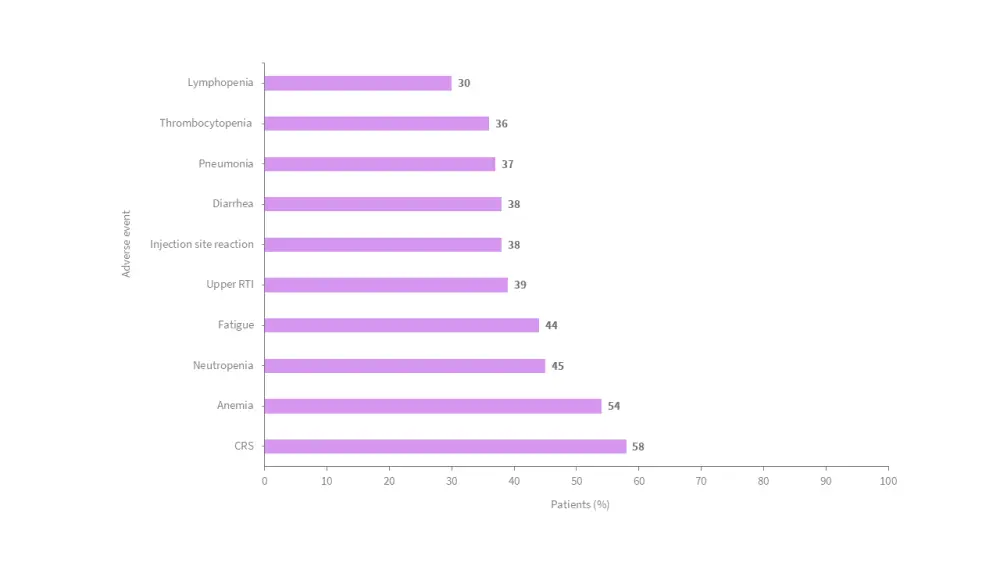
CRS, cytokine release syndrome; RTI, respiratory tract infection.
*Adapted from Pfizer, 2023
The European Commission is due to make a legally binding decision in the coming months. If granted, this decision will apply to all 27 member states. Ongoing trials, such as the MagnetisMM-5 (NCT05020236), -6 (NCT05623020), and -7 (NCT05317416), are exploring the use of elranatamab as a monotherapy and in combination with standard or novel therapies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



