All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Multiple myeloma (MM) and other monoclonal gammopathies can increase patient susceptibility to infection, either as a direct result of disease characteristics and comorbidities, or treatment-mediated immune suppression. With regard to the COVID-19 pandemic, this can translate to prolonged infection and an increased risk of mortality following infection with SARS-CoV-2.1 In a recent podcast, Michel Delforge and Noopur Raje highlighted the increased vulnerability of patients with MM to infections, including COVID-19.
Historically, patients with MM also demonstrate suboptimal vaccine-mediated antibody responses. As a result, a number of studies are evaluating anti-SARS-CoV-2 antibody perseverance in patients with MM following infection and vaccination. Although ongoing, data from these studies suggest a suboptimal SARS-CoV-2 antibody response in patients with MM, highlighting a need for close monitoring and clinical management.1
In contrast to MM, data from the iStopMM study (N = 75,422) suggest that monoclonal gammopathy of undetermined significance (MGUS) is not associated with an increased risk of SARS-CoV-2 infection and COVID-19 severity. This important finding highlights immune response variation between patients with MM and MGUS, influencing guidelines on the treatment and management of these patients moving forward.2
A review by Heinz Ludwig and colleagues1 consolidated the latest European Multiple Myeloma Network (EMN) consensus guidelines on COVID-19 vaccination in patients with MM, below is a summary.
Treatment
Anti-myeloma treatments, particularly anti-CD38 therapies, can impair patient immune responses. Limiting immunosuppression in patients undergoing treatment for MM and other monoclonal gammopathies has become ever more pressing throughout the COVID-19 era. There are a number of studies evaluating approaches to reduce the risk of severe COVID-19 disease in these patients, including over 560 clinical trials evaluating active treatments for COVID-19. The recommended treatment adaptations and key considerations are outlined in Figures 1 & 2.
Figure 1. Adaptations of treatments for patients with monoclonal gammopathies throughout the COVID-19 pandemic*
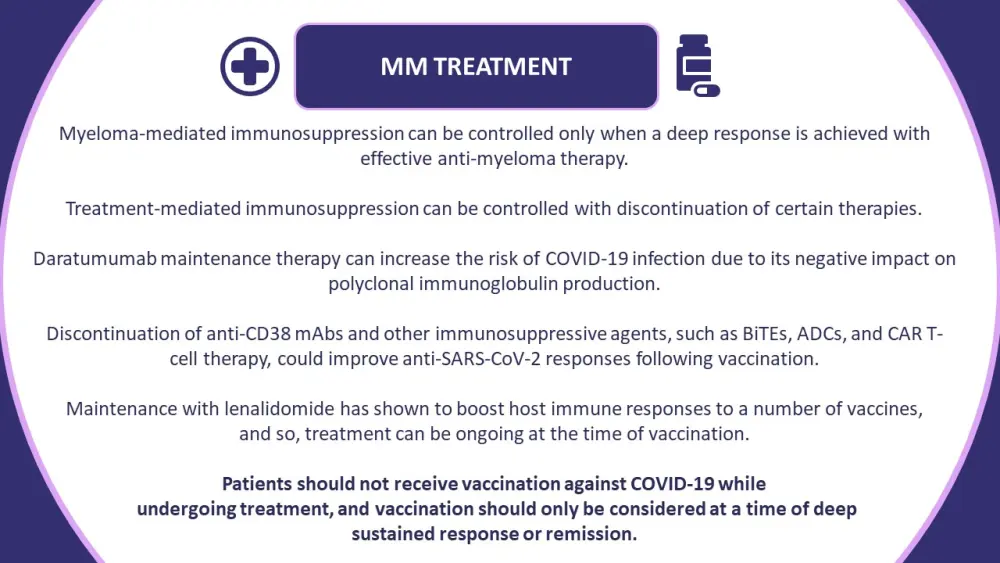
ADCs, antibody-drug conjugates; BiTEs, bispecific T-cell engagers; CAR, chimeric antigen receptor; mAb, monoclonal antibody; MM, multiple myeloma.
*Information from Ludwig, et al.1
Figure 2. Guidelines on the prevention and treatment of COVID-19 in patients with monoclonal gammopathies*
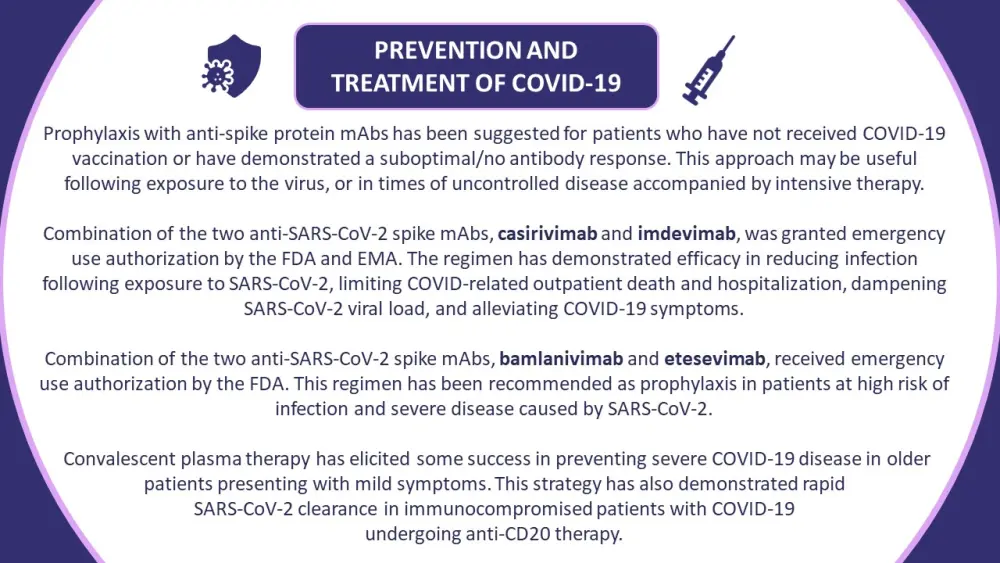
EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; mAbs, monoclonal antibodies.
*Information from Ludwig, et al.1
Vaccination
Real-world data suggest that patients with MM are generally willing to receive a COVID-19 vaccine, more so than the general population. The EMN has set out vaccination guidelines for healthcare professionals involved in the treatment and care of patients with MGUS, smoldering multiple myeloma, multiple myeloma, and monoclonal gammopathies of clinical significance (Figure 3). It has been recommended that all patients with these conditions be vaccinated against COVID-19.
The guidelines recognize uncontrolled disease, immunoparesis, >1 previous line of therapy, specific treatment classes, and older age as risk factors for inferior responses to COVID-19 vaccination. Despite not receiving backing from the Centers for Disease Control and Prevention, analyzing patient vaccine responses is crucial to identify those with suboptimal anti-SARS-CoV-2 responses and who might benefit from a third dose.
Figure 3. Guidelines on the vaccination of patients with monoclonal gammopathies against SARS-CoV-2*
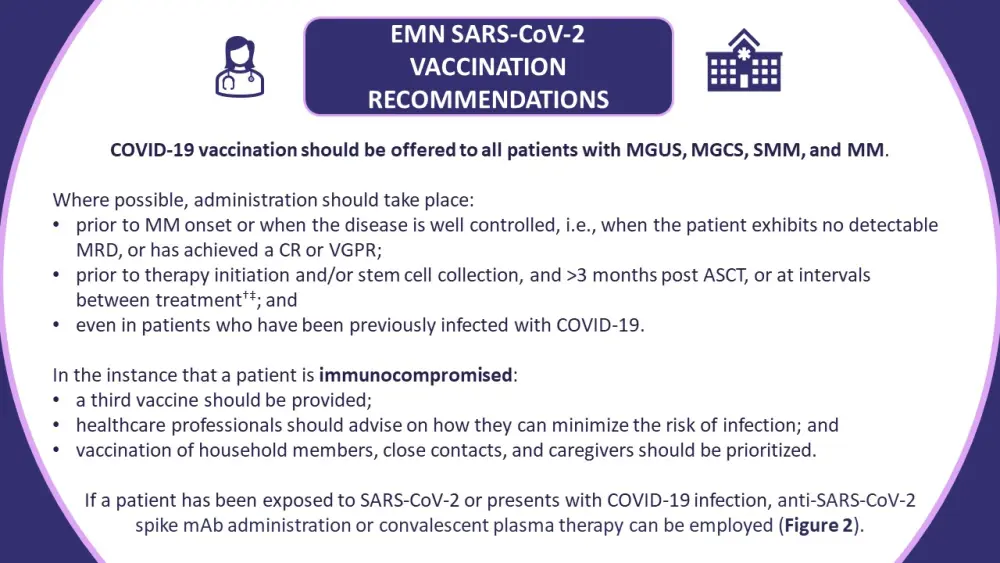
ASCT, autologous stem cell transplantation; CR, complete response; EMN, European Multiple Myeloma Network; mAb, monocolonal antibody; MGCS, monoclonal gammopathy of clinical significance; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; MRD, measurable residual disease; SMM, smoldering multiple myeloma; VGPR, very good partial response.
*Information from Ludwig, et al.1
†Lenalidomide maintenance therapy is an exception.
‡Vaccination should be considered on an individual basis to account for patients with poorly controlled disease undergoing continuous therapy.
Conclusion
The EMN COVID-19 consensus recommendations provide guidance around the vaccination strategy for patients with monoclonal gammopathies. The guidance also covers the treatment and management of both the underlying disease and COVID-19 in these patients, with the aim to reduce the risk of infection, severe disease, and mortality. The Multiple Myeloma Hub was pleased to receive comment from primary author, Heinz Ludwig:
Expert Opinion
Many myeloma patients have an increased risk for COVID-19 infections, a prolonged course of infection, and mortality. Hence, monitoring the immune response to vaccination and adapting management such as recommendation of additional vaccine doses, and social distancing is important. Further options are the administration of monoclonal antibody cocktails and hopefully soon treatment and prophylaxis with antiviral drugs.
 Heinz Ludwig
Heinz LudwigReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

